HLA-E
HLA class I histocompatibility antigen, alpha chain E (HLA-E) also known as MHC class I antigen E is a protein that in humans is encoded by the HLA-E gene.[1] The human HLA-E is a non-classical MHC class I molecule that is characterized by a limited polymorphism and a lower cell surface expression than its classical paralogues. The functional homolog in mice is called Qa-1b.
Structure
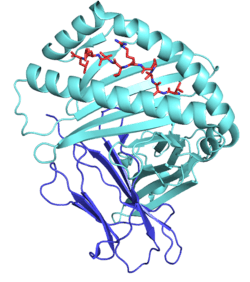
Like other MHC class I molecules, HLA-E is a heterodimer consisting of an α heavy chain and a light chain (β-2 microglobulin). The heavy chain is approximately 45 kDa and anchored in the membrane. The HLA-E gene contains 8 exons. Exon one encodes the signal peptide, exons 2 and 3 encode the α1 and α2 domains, which both bind the peptide, exon 4 encodes the α3 domain, exon 5 encodes the transmembrane domain, and exons 6 and 7 encode the cytoplasmic tail.[2]
Function
HLA-E has a very specialized role in cell recognition by natural killer cells (NK cells).[3] HLA-E binds a restricted subset of peptides derived from signal peptides of classical MHC class I molecules, namely HLA-A, B, C, G.[4] These peptides are released from the membrane of the endoplasmic reticulum (ER) by the signal peptide peptidase and trimmed by the cytosolic proteasome.[5][6] Upon transport into the ER lumen by the transporter associated with antigen processing (TAP), these peptides bind to a peptide binding groove on the HLA-E molecule.[7] This allows HLA-E to assemble correctly and to be expressed on the cell surface. NK cells recognize the HLA-E+peptide complex using the heterodimeric inhibitory receptor CD94/NKG2A/B/C.[3] When CD94/NKG2A or CD94/NKG2B is engaged, it produces an inhibitory effect on the cytotoxic activity of the NK cell to prevent cell lysis. However, binding of HLA-E to CD94/NKG2C results in NK cell activation. This interaction has been shown to trigger expansion of NK cell subsets in antiviral responses.[8]
References
- ↑ Mizuno S, Trapani JA, Koller BH, Dupont B, Yang SY (Jun 1988). "Isolation and nucleotide sequence of a cDNA clone encoding a novel HLA class I gene". Journal of Immunology 140 (11): 4024–30. PMID 3131426.
- ↑ "Entrez Gene: HLA-E major histocompatibility complex, class I, E".
- ↑ 3.0 3.1 Braud VM, Allan DS, O'Callaghan CA, Söderström K, D'Andrea A, Ogg GS et al. (Feb 1998). "HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C". Nature 391 (6669): 795–9. doi:10.1038/35869. PMID 9486650.
- ↑ Braud V, Jones EY, McMichael A (May 1997). "The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9". European Journal of Immunology 27 (5): 1164–9. doi:10.1002/eji.1830270517. PMID 9174606.
- ↑ Lemberg MK, Bland FA, Weihofen A, Braud VM, Martoglio B (Dec 2001). "Intramembrane proteolysis of signal peptides: an essential step in the generation of HLA-E epitopes". Journal of Immunology 167 (11): 6441–6. doi:10.4049/jimmunol.167.11.6441. PMID 11714810.
- ↑ Bland FA, Lemberg MK, McMichael AJ, Martoglio B, Braud VM (Sep 2003). "Requirement of the proteasome for the trimming of signal peptide-derived epitopes presented by the nonclassical major histocompatibility complex class I molecule HLA-E". The Journal of Biological Chemistry 278 (36): 33747–52. doi:10.1074/jbc.M305593200. PMID 12821659.
- ↑ Braud VM, Allan DS, Wilson D, McMichael AJ (Jan 1998). "TAP- and tapasin-dependent HLA-E surface expression correlates with the binding of an MHC class I leader peptide". Current Biology : CB 8 (1): 1–10. doi:10.1016/S0960-9822(98)70014-4. PMID 9427624.
- ↑ Rölle A, Pollmann J, Ewen EM, Le VT, Halenius A, Hengel H et al. (Dec 2014). "IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion". The Journal of Clinical Investigation 124 (12): 5305-16. doi:10.1172/JCI77440. PMID 25384219.
Further reading
- Kuby Immunology, 6th edition, by Thomas J. Kindt, Richard A. Goldsby,and Barbara A.Kuby W.H. Freeman and Company,New York
- Moretta L, Bottino C, Pende D, Mingari MC, Biassoni R, Moretta A (May 2002). "Human natural killer cells: their origin, receptors and function". European Journal of Immunology 32 (5): 1205–11. doi:10.1002/1521-4141(200205)32:5<1205::AID-IMMU1205>3.0.CO;2-Y. PMID 11981807.
- Jensen PE, Sullivan BA, Reed-Loisel LM, Weber DA (Jun 2004). "Qa-1, a nonclassical class I histocompatibility molecule with roles in innate and adaptive immunity". Immunologic Research 29 (1-3): 81–92. doi:10.1385/IR:29:1-3:081. PMID 15181272.
PDB gallery |
|---|
| | 1kpr: The human non-classical major histocompatibility complex molecule HLA-E |
| 1ktl: The human non-classical major histocompatibility complex molecule HLA-E |
| 1mhe: THE HUMAN NON-CLASSICAL MAJOR HISTOCOMPATIBILITY COMPLEX MOLECULE HLA-E |
| 2esv: Structure of the HLA-E-VMAPRTLIL/KK50.4 TCR complex |
|
|
|
|
|---|
| Major histocompatibility complex/
Human leukocyte antigen | |
|---|
| | Other | |
|---|
| |
|---|
| | Description |
- Physiology
- cells
- autoantigens
- autoantibodies
- complement
- surface antigens
- IG receptors
|
|---|
| | Disease |
- Allergies
- Immunodeficiency
- Immunoproliferative immunoglobulin disorders
- Hypersensitivity and autoimmune disorders
- Neoplasms and cancer
|
|---|
| | Treatment |
- Procedures
- Drugs
- antihistamines
- immunostimulants
- immunosuppressants
- monoclonal antibodies
|
|---|
|
|