HFE hereditary haemochromatosis
| Haemochromatosis type 1 | |
|---|---|
| Classification and external resources | |
| ICD-10 | E83.1 |
| ICD-9 | 275.0 |
| OMIM | 235200 |
| DiseasesDB | 5490 |
| eMedicine | med/975 derm/878 |
| MeSH | D006432 |
| GeneReviews | |
Haemochromatosis (or hemochromatosis) type 1[1] (also HFE hereditary haemochromatosis[2] or HFE-related hereditary haemochromatosis[3]) is a hereditary disease characterized by excessive intestinal absorption of dietary iron resulting in a pathological increase in total body iron stores.[4] Humans, like most animals, have no means to excrete excess iron.[5] Excess iron accumulates in tissues and organs disrupting their normal function. The most susceptible organs include the liver, adrenal glands, heart, skin, gonads, joints, and the pancreas; patients can present with cirrhosis, polyarthropathy, adrenal insufficiency, heart failure or diabetes.[6] The hereditary form of the disease is most common among those of Northern European ancestry, in particular those of Celtic descent.[7] The disease is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations.[8] Most often, the parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but do not show signs and symptoms of the condition.[8]
Signs and symptoms
Haemochromatosis is protean in its manifestations, i.e., often presenting with signs or symptoms suggestive of other diagnoses that affect specific organ systems. Many of the signs and symptoms below are uncommon and most patients with the hereditary form of haemochromatosis do not show any overt signs of disease nor do they suffer premature morbidity.[9]
The classic triad of cirrhosis, bronze skin and diabetes is not as common any more because of earlier diagnosis.[10]
The more common clinical manifestations include:[6][10][11][12]
- Fatigue
- Malaise
- Joint and bone pain
- Liver cirrhosis (with an increased risk of hepatocellular carcinoma) Liver disease is always preceded by evidence of liver dysfunction including elevated serum enzymes specific to the liver, clubbing of the fingers, leuconychia, asterixis, hepatomegaly, palmar erythema and spider naevi. Cirrhosis can also present with jaundice (yellowing of the skin) and ascites.
- Insulin resistance (often patients have already been diagnosed with diabetes mellitus type 2) due to pancreatic damage from iron deposition
- Erectile dysfunction and hypogonadism, resulting in decreased libido
- Congestive heart failure, arrhythmias or pericarditis
- Arthritis of the hands (especially the second and third MCP joints), but also the knee and shoulder joints
- Damage to the adrenal gland, leading to adrenal insufficiency
Less common findings including:
- Deafness[13]
- Dyskinesias, including Parkinsonian symptoms[13][14][15]
- Dysfunction of certain endocrine organs:
- Parathyroid gland (leading to hypocalcaemia)
- Pituitary gland
- More commonly a slate-grey or less commonly darkish colour to the skin (see pigmentation, hence its name "diabetes bronze" when it was first described by Armand Trousseau in 1865)
- An increased susceptibility to certain infectious diseases caused by siderophilic microorganisms:
- Vibrio vulnificus infections from eating seafood or wound infection[16]
- Listeria monocytogenes
- Yersinia enterocolica
- Salmonella enterica (serotype Typhymurium)[17]
- Klebsiella pneumoniae
- Escherichia coli
- Rhizopus arrhizus
- Mucor species
Males are usually diagnosed after their forties and fifties, and women several decades later, owing to regular iron loss through menstruation (which ceases in menopause). The severity of clinical disease in the hereditary form varies considerably. There is evidence suggesting that hereditary haemochromatosis patients affected with other liver ailments such as hepatitis or alcoholic liver disease suffer worse liver disease than those with either condition alone. There are also juvenile forms of hereditary haemochromatosis that present in childhood with the same consequences of iron overload.
End-organ damage
Iron is stored in the liver, the pancreas and the heart. Long-term effects of haemochromatosis on these organs can be very serious, even fatal when untreated.[18] For example, similar to alcoholism, haemochromatosis can cause cirrhosis of the liver. The liver is a primary storage area for iron and will naturally accumulate excess iron. Over time the liver is likely to be damaged by iron overload. Cirrhosis itself may lead to additional and more serious complications, including bleeding from dilated veins in the esophagus (esophageal varices) and stomach (gastric varices) and severe fluid retention in the abdomen (ascites). Toxins may accumulate in the blood and eventually affect mental functioning. This can lead to confusion or even coma (hepatic encephalopathy).
Liver cancer: Cirrhosis and haemochromatosis together will increase the risk of liver cancer. (Nearly one-third of people with haemochromatosis and cirrhosis eventually develop liver cancer.)
Diabetes: The pancreas which also stores iron is very important in the body’s mechanisms for sugar metabolism. Diabetes affects the way the body uses blood sugar (glucose). Diabetes is in turn the leading cause of new blindness in adults and may be involved in kidney failure and cardiovascular disease.
Congestive heart failure: If excess iron in the heart interferes with the its ability to circulate enough blood, a number of problems can occur including death. The condition may be reversible when haemochromatosis is treated and excess iron stores reduced.
Heart arrhythmias: Arrhythmia or abnormal heart rhythms can cause heart palpitations, chest pain and light-headedness and are occasionally life-threatening. This condition can often be reversed with treatment for haemochromatosis.
Pigment changes: Bronze or grey coloration of the skin is caused primarily by increased melanin deposition, with iron deposition playing a lesser role.[19]
Genetics
The regulation of dietary iron absorption is complex and our understanding is incomplete. One of the better characterized genes responsible for hereditary haemochromatosis is HFE[20] on chromosome 6 which codes for a protein that participates in the regulation of iron absorption. The HFE gene has two common mutations, C282Y and H63D.[21] The C282Y allele is a transition point mutation from guanine to adenine at nucleotide 845 in the HFE gene, resulting in a missense mutation that replaces the cysteine residue at position 282 with a tyrosine amino acid.[22] Heterozygotes for either allele do not manifest clinical iron overload but may display an increased iron uptake. Mutations of the HFE gene account for 90% of the cases of non-transfusional iron overload. This gene is closely linked to the HLA-A3 locus. Homozygosity for the C282Y mutation is the most common genotype responsible for clinical iron accumulation, though heterozygosity for C282Y/H63D mutations, so-called compound heterozygotes, results in clinically evident iron overload. There is considerable debate regarding the penetrance—the probability of clinical expression of the trait given the genotype—is for clinical disease in HHC homozygotes. Most, if not all, males homozygous for HFE C282Y will show manifestations of liver dysfunction such as elevated liver-specific enzymes such as serum gamma glutamyltransferase (GGT) by late middle age. Homozygous females can delay the onset of iron accumulation because of iron loss through menstruation. Each patient with the susceptible genotype accumulates iron at different rates depending on iron intake, the exact nature of the mutation and the presence of other insults to the liver such as alcohol and viral disease. As such the degree to which the liver and other organs is affected, expressivity, is highly variable and is dependent on such these other factors and co-morbidities as well as age at which they are studied for manifestations of disease.[23] Penetrance differs between different populations.
Pathophysiology
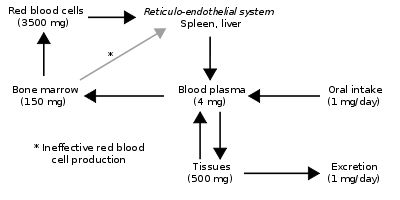
Since the regulation of iron metabolism is still poorly understood, a clear model of how haemochromatosis operates is still not available. A working model describes the defect in the HFE gene, where a mutation puts the intestinal absorption of iron into overdrive. Normally, HFE facilitates the binding of transferrin, which is iron's carrier protein in the blood. Transferrin levels are typically elevated at times of iron depletion (low ferritin stimulates the release of transferrin from the liver). When transferrin is high, HFE works to increase the intestinal release of iron into the blood. When HFE is mutated, the intestines perpetually interpret a strong transferrin signal as if the body were deficient in iron. This leads to maximal iron absorption from ingested foods and iron overload in the tissues. However, HFE is only part of the story, since many patients with mutated HFE do not manifest clinical iron overload, and some patients with iron overload have a normal HFE genotype. A possible explanation is the fact that HFE normally plays a role in the production of hepcidin in the liver, a function that is impaired in HFE mutations.[24]
People with abnormal iron regulatory genes do not reduce their absorption of iron in response to increased iron levels in the body. Thus the iron stores of the body increase. As they increase, the iron which is initially stored as ferritin is deposited in organs as haemosiderin and this is toxic to tissue, probably at least partially by inducing oxidative stress.[25] Iron is a pro-oxidant. Thus, haemochromatosis shares common symptomology (e.g., cirrhosis and dyskinetic symptoms) with other "pro-oxidant" diseases such as Wilson's disease, chronic manganese poisoning, and hyperuricaemic syndrome in Dalmatian dogs. The latter also experience "bronzing".
Diagnosis
The diagnosis of haemochromatosis is often made following the incidental finding on routine blood screening of elevated serum liver enzymes or elevation of the transferrin saturation. Arthropathy with stiff joints, diabetes, or fatigue, may be the presenting complaint.[26]
Blood tests
Serum transferrin and transferrin saturation are commonly used as screening for haemochromatosis. Transferrin binds iron and is responsible for iron transport in the blood.[27] Measuring transferrin provides a crude measure of iron stores in the body. Fasting transferrin saturation values in excess of 45% (or 35% in premenopausal women) are recognized as a threshold for further evaluation of haemochromatosis.[10] Transferrin saturation greater than 62% is suggestive of homozygosity for mutations in the HFE gene.[28]
Serum Ferritin: Ferritin, a protein synthesized by the liver is the primary form of iron storage within cells and tissues. Measuring ferritin provides another crude estimate of whole body iron stores though many conditions, particularly inflammation (but also chronic alcohol consumption, non-alcoholic fatty liver disease and others), can elevate serum ferritin - which can account for up to 90% of cases where elevated levels are observed.[4] Normal values for males are 12–300 ng/ml (nanograms per millilitre) and for female, 12–150 ng/ml.[26][29] Serum ferritin in excess of 1000 nanograms per millilitre of blood is almost always attributable to haemochromatosis.
Other blood tests routinely performed: blood count, renal function, liver enzymes, electrolytes, glucose (and/or an oral glucose tolerance test (OGTT)).
Liver biopsy

Liver biopsies involve taking a sample of tissue from the liver, using a thin needle. The amount of iron in the sample is then quantified and compared to normal, and evidence of liver damage, especially cirrhosis, measured microscopically. Formerly, this was the only way to confirm a diagnosis of haemochromatosis but measures of transferrin and ferritin along with a history are considered adequate in determining the presence of the malady. Risks of biopsy include bruising, bleeding and infection. Now, when a history and measures of transferrin or ferritin point to haemochromatosis, it is debatable whether a liver biopsy is still necessary to quantify the amount of accumulated iron.[26]
MRI
MRI-based testing is a non-invasive and accurate alternative to measure liver iron concentrations.[30]
Other imaging
Clinically the disease may be silent, but characteristic radiological features may point to the diagnosis. The increased iron stores in the organs involved, especially in the liver and pancreas, result in characteristic findings on unenhanced CT and a decreased signal intensity in MRI scans. Haemochromatosis arthropathy includes degenerative osteoarthritis and chondrocalcinosis. The distribution of the arthropathy is distinctive, but not unique, frequently affecting the second and third metacarpophalangeal joints of the hand. The arthropathy can therefore be an early clue as to the diagnosis of haemochromatosis.
Functional testing
Based on the history, the doctor might consider specific tests to monitor organ dysfunction, such as an echocardiogram for heart failure, or blood glucose monitoring for patients with haemochromatosis diabetes.
Differential diagnosis
There exist other causes of excess iron accumulation, which have to be considered before haemochromatosis is diagnosed.
- African iron overload, formerly known as Bantu siderosis, was first observed among people of African descent in Southern Africa. Originally, this was blamed on ungalvanised barrels used to store home-made beer, which led to increased oxidation and increased iron levels in the beer. Further investigation has shown that only some people drinking this sort of beer get an iron overload syndrome, and that a similar syndrome occurred in people of African descent who have had no contact with this kind of beer (e.g., African Americans). This led investigators to the discovery of a gene polymorphism in the gene for ferroportin which predisposes some people of African descent to iron overload.[31]
- Transfusion haemosiderosis is the accumulation of iron, mainly in the liver, in patients who receive frequent blood transfusions (such as those with thalassaemia).
- Dyserythropoeisis, also known as myelodysplastic syndrome, is a disorder in the production of red blood cells. This leads to increased iron recycling from the bone marrow and accumulation in the liver.
Screening
Standard diagnostic measures for haemochromatosis, transferrin saturation and ferritin tests, are not a part of routine medical testing. Screening for haemochromatosis is recommended if the patient has a parent, child or sibling with the disease.[32]
Routine screening of the general population for hereditary haemochromatosis is generally not done. Mass genetic screening has been evaluated by the U.S. Preventive Services Task Force (USPSTF), among other groups. The USPSTF recommended against genetic screening of the general population for hereditary haemochromatosis because the likelihood of discovering an undiagnosed patient with clinically relevant iron overload is less than 1 in 1,000. Although there is strong evidence that treatment of iron overload can save lives in patients with transfusional iron overload, no clinical study has shown that for asymptomatic carriers of hereditary haemochromatosis treatment with venesection (phlebotomy) provides any clinical benefit.[33][34] Recently, it has been suggested that patients be screened for iron overload using serum ferritin as a marker: If serum ferritin exceeds 1000 ng/mL, iron overload is very likely the cause.
Treatment
Phlebotomy
Early diagnosis is important because the late effects of iron accumulation can be wholly prevented by periodic phlebotomies (by venesection) comparable in volume to blood donations.[35] Treatment is initiated when ferritin levels reach 300 nanograms per millilitre (or 200 in nonpregnant premenopausal women).
Phlebotomy (or bloodletting) is usually done at a weekly interval until ferritin levels are less than 20 milligrams per litre. After that, 1–6 donations per year are usually needed to maintain iron balance.
Desferrioxamine mesilate
Where venesection is not possible, long-term administration of desferrioxamine mesilate is useful. Desferrioxamine is an iron-chelating compound, and excretion induced by desferrioxamine is enhanced by administration of Vitamin C. It cannot be used during pregnancy or breast-feeding due to risk of defects in the child.
Organ damage
- Treatment of organ damage (heart failure with diuretics and ACE inhibitor therapy).
Diet
- Limiting intake of alcoholic beverages, vitamin C (increases iron absorption in the gut), red meat (high in iron) and potential causes of food poisoning (shellfish, seafood).
- Increasing intake of substances that inhibit iron absorption, such as high-tannin tea, calcium, and foods containing oxalic and phytic acids (such as collard greens, which must be consumed at the same time as the iron-containing foods in order to be effective).[36]
Epidemiology
Haemochromatosis is one of the most common heritable genetic conditions in people of northern European extraction with a prevalence of 1 in 200. The disease has a variable penetration and about 1 in 10 people of this demographic carry a mutation in one of the genes regulating iron metabolism, the most common allele being the C282Y allele in the HFE gene.[37] The prevalence of mutations in iron metabolism genes varies in different populations. A study of 3,011 unrelated white Australians found that 14% were heterozygous carriers of an HFE mutation, 0.5% were homozygous for an HFE mutation, and only 0.25% of the study population had clinically relevant iron overload. Most patients who are homozygous for HFE mutations will not manifest clinically relevant haemochromatosis (see Genetics above).[23] Other populations have a lower prevalence of both the genetic mutation and the clinical disease.
Genetic studies suggest the original haemochromatosis mutation arose in a single person, possibly of Celtic ethnicity, who lived 60–70 generations ago.[38] At that time when dietary iron may have been scarcer than today, the presence of the mutant allele may have provided an evolutionary or natural selection reproductive advantage by maintaining higher iron levels in the blood.
Terminology
The term "haemochromatosis" is used by different sources in many different ways.
It is often used to imply an association with the HFE gene. For many years, HFE was the only known gene associated with haemochromatosis, and the term "hereditary haemochromatosis" was used to describe haemochromatosis type 1. However, it is now known that there are many different genetic associations with this condition. The older the text, or the more general the audience, the more likely that HFE is implied.
"Haemochromatosis" has also been used in contexts where there had not been a known genetic cause for iron accumulation. In some cases, however, a condition that was thought to be due to diet or environment was later linked to a genetic polymorphism, as in African iron overload.
History
The disease was first described in 1865 by Armand Trousseau in a report on diabetes in patients presenting with a bronze pigmentation of their skin.[39] Trousseau did not associate diabetes with iron accumulation; the recognition that infiltration of the pancreas with iron might disrupt endocrine function resulting in diabetes was made by Friedrich Daniel von Recklinghausen in 1890.[40][41]
References
- ↑ Franchini M (March 2006). "Hereditary iron overload: update on pathophysiology, diagnosis, and treatment". Am. J. Hematol. 81 (3): 202–9. doi:10.1002/ajh.20493. PMID 16493621.
- ↑ Allen KJ, Gurrin LC, Constantine CC et al. (January 2008). "Iron-overload-related disease in HFE hereditary hemochromatosis". N. Engl. J. Med. 358 (3): 221–30. doi:10.1056/NEJMoa073286. PMID 18199861.
- ↑ Jacobs EM, Verbeek AL, Kreeftenberg HG et al. (December 2007). "Changing aspects of HFE-related hereditary haemochromatosis and endeavours to early diagnosis". Neth J Med 65 (11): 419–24. PMID 18079564.
- ↑ 4.0 4.1 St John, Andrew. "Testing for HFE-related haemochromatosis". Australian Prescriber (34): 73–6.
- ↑ "The interaction of iron and erythropoietin".
- ↑ 6.0 6.1 Iron Overload and Hemochromatosis Centers for Disease Control and Prevention
- ↑ "Celtic Curse".
- ↑ 8.0 8.1 "hemochromatosis".
- ↑ Hemochromatosis-Diagnosis National Digestive Diseases Information Clearinghouse, National Institutes of Health, U.S. Department of Health and Human Services
- ↑ 10.0 10.1 10.2 Pietrangelo A (June 2004). "Hereditary hemochromatosis—a new look at an old disease". N. Engl. J. Med. 350 (23): 2383–97. doi:10.1056/NEJMra031573. PMID 15175440.
- ↑ Hemochromatosis National Digestive Diseases Information Clearinghouse, National Institutes of Health, U.S. Department of Health and Human Services
- ↑ "Hemochromatosis: Symptoms". Mayo Foundation for Medical Education and Research (MFMER).
- ↑ 13.0 13.1 Jones H, Hedley-Whyte E (1983). "Idiopathic hemochromatosis (IHC): dementia and ataxia as presenting signs". Neurology 33 (11): 1479–83. doi:10.1212/WNL.33.11.1479. PMID 6685241.
- ↑ Costello D, Walsh S, Harrington H, Walsh C (2004). "Concurrent hereditary haemochromatosis and idiopathic Parkinson's disease: a case report series". J Neurol Neurosurg Psychiatry 75 (4): 631–3. doi:10.1136/jnnp.2003.027441. PMC 1739011. PMID 15026513.
- ↑ Nielsen J, Jensen L, Krabbe K (1995). "Hereditary haemochromatosis: a case of iron accumulation in the basal ganglia associated with a parkinsonian syndrome". J Neurol Neurosurg Psychiatry 59 (3): 318–21. doi:10.1136/jnnp.59.3.318. PMC 486041. PMID 7673967.
- ↑ Barton JC, Acton RT (April 2009). "Hemochromatosis and Vibrio vulnificus Wound Infections". J. Clin. Gastroenterol. 43 (9): 890–893. doi:10.1097/MCG.0b013e31819069c1. PMID 19349902.
- ↑ Jolivet-Gougeon A, Loréal O, Ingels A et al. (October 2008). "Serum transferrin saturation increase is associated with decrease of antibacterial activity of serum in patients with HFE-related genetic hemochromatosis". Am. J. Gastroenterol. 103 (10): 2502–8. doi:10.1111/j.1572-0241.2008.02036.x. PMID 18684194.
- ↑ "Hemochromatosis: Complications". Mayo Foundation for Medical Education and Research (MFMER).
- ↑ Pedrup A, Poulsen H (1964). "Hemochromatosis and Vitiligo". Archives of Dermatology 90 (1): 34–37. doi:10.1001/archderm.1964.01600010040010. PMID 14149720.
- ↑ Olynyk JK, Trinder D, Ramm GA, Britton RS, Bacon BR (September 2008). "Hereditary hemochromatosis in the post-HFE era". Hepatology 48 (3): 991–1001. doi:10.1002/hep.22507. PMC 2548289. PMID 18752323.
- ↑ "Hemochromatosis: Causes". Mayo Foundation for Medical Education and Research (MFMER).
- ↑ Feder JN, Gnirke A, Thomas W et al. (1996). "A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis". Nature Genetics 13 (4): 399–408. doi:10.1038/ng0896-399. PMID 8696333.
- ↑ 23.0 23.1 Olynyk J, Cullen D, Aquilia S, Rossi E, Summerville L, Powell L (1999). "A population-based study of the clinical expression of the hemochromatosis gene". N Engl J Med 341 (10): 718–24. doi:10.1056/NEJM199909023411002. PMID 10471457.
- ↑ Vujić Spasić M, Kiss J, Herrmann T et al. (2008). "Hfe acts in hepatocytes to prevent hemochromatosis". Cell Metab. 7 (2): 173–8. doi:10.1016/j.cmet.2007.11.014. PMID 18249176.
- ↑ Shizukuda Y, Bolan C, Nguyen T, Botello G, Tripodi D, Yau Y, Waclawiw M, Leitman S, Rosing D (2007). "Oxidative stress in asymptomatic subjects with hereditary hemochromatosis". Am J Hematol 82 (3): 249–50. doi:10.1002/ajh.20743. PMID 16955456.
- ↑ 26.0 26.1 26.2 "Hemochromatosis: Tests and diagnosis". Mayo Foundation for Medical Education and Research (MFMER). Retrieved 2009-04-20.
- ↑ Transferrin and Iron Transport Physiology
- ↑ Dadone MM, Kushner JP, Edwards CQ, Bishop DT, Skolnick MH (August 1982). "Hereditary hemochromatosis. Analysis of laboratory expression of the disease by genotype in 18 pedigrees". American Journal of Clinical Pathology 78 (2): 196–207. PMID 7102818.
- ↑ MedlinePlus Encyclopedia Ferritin Test Measuring iron in the body
- ↑ St Pierre; Clark, PR; Chua-Anusorn, W; Fleming, AJ; Jeffrey, GP; Olynyk, JK; Pootrakul, P; Robins, E; Lindeman, R (2005). "Non-invasive measurement and imaging of liver iron concentrations using proton magnetic resonance". Blood. 105 (2): 855–61. doi:10.1182/blood-2004-01-0177. PMID 15256427.
- ↑ Gordeuk V, Caleffi A, Corradini E, Ferrara F, Jones R, Castro O, Onyekwere O, Kittles R, Pignatti E, Montosi G, Garuti C, Gangaidzo I, Gomo Z, Moyo V, Rouault T, MacPhail P, Pietrangelo A (2003). "Iron overload in Africans and African-Americans and a common mutation in the SCL40A1 (ferroportin 1) gene". Blood Cells Mol Dis 31 (3): 299–304. doi:10.1016/S1079-9796(03)00164-5. PMID 14636642.
- ↑ "Summaries for patients. Screening for hereditary hemochromatosis: recommendations from the American College of Physicians". Ann. Intern. Med. 143 (7): I46. 2005. doi:10.7326/0003-4819-143-7-200510040-00004. PMID 16204158.
- ↑ U.S. Preventive Services Task Force (2006). "Screening for haemochromatosis: recommendation statement". Ann. Intern. Med. 145 (3): 204–8. doi:10.7326/0003-4819-145-3-200608010-00008. PMID 16880462.
- ↑ Screening for Hemochromatosis U.S. Preventive Services Task Force (2006). Summary of Screening Recommendations and Supporting Documents. Retrieved 18 March 2007
- ↑ "Hemochromatosis: Treatments and drugs". Mayo Foundation for Medical Education and Research (MFMER).
- ↑ http://dynaweb.ebscohost.com/Detail.aspx?id=116469&sid=14aa79e5-a881-407c-94e7-339b81c4cd18@sessionmgr3 accessed October 15, 2008.
- ↑ Mendes AI, Ferro A, Martins R et al. (March 2009). "Non-classical hereditary hemochromatosis in Portugal: novel mutations identified in iron metabolism-related genes". Ann. Hematol. 88 (3): 229–34. doi:10.1007/s00277-008-0572-y. PMID 18762941.
- ↑ http://www.scripps.edu/bcmd/pdfarea/issue_20_98/lucotte.pdf
- ↑ Trousseau A (1865). "Glycosurie, diabète sucré". Clinique médicale de l'Hôtel-Dieu de Paris 2: 663–98.
- ↑ von Recklinghausen FD (1890). "Hämochromatose". Tageblatt der Naturforschenden Versammlung 1889: 324.
- ↑ Biography of Daniel von Recklinghausen
External links
- GeneReview/NIH/UW entry on HFE-Associated Hereditary Hemochromatosis
- HFE hereditary haemochromatosis at DMOZ
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||