Gynoecium




Gynoecium (from Ancient Greek γυνή, gyne, meaning woman, and οἶκος, oikos, meaning house) is most commonly used as a collective term for the parts of a flower that produce ovules and ultimately develop into the fruit and seeds. The gynoecium is the innermost whorl of (one or more) pistils in a flower and is typically surrounded by the pollen-producing reproductive organs, the stamens, collectively called the androecium. The gynoecium is often referred to as the "female" portion of the flower, although rather than directly producing female gametes (i.e. egg cells), the gynoecium produces megaspores, each of which develops into a female gametophyte which then produces egg cells.
The term gynoecium is also used by botanists to refer to a cluster of archegonia and any associated modified leaves or stems present on a gametophyte shoot in mosses, liverworts and hornworts.
Flowers that bear a gynoecium but no androecium are called carpellate. Flowers lacking a gynoecium are called staminate.
The gynoecium is often referred to as female because it gives rise to female (egg-producing) gametophytes, however, strictly speaking sporophytes do not have sex, only gametophytes do.[1]
Gynoecium development and arrangement is important in systematic research and identification of angiosperms, but can be the most challenging of the floral parts to interpret.[2]
Pistils

The gynoecium may consist of one or more separate pistils. A pistil typically consists of an expanded basal portion called the ovary, an elongated section called a style and an apical structure that receives pollen called a stigma.
- The ovary (from Latin ovum meaning egg), is the enlarged basal portion which contains placentas, ridges of tissue bearing one or more ovules (integumented megasporangia). The placentas and/or ovule(s) may be born on the gynoecial appendages or less frequently on the floral apex.[3][4][5][6][7] The chamber in which the ovules develop is called a locule (or sometimes cell).
- The style (from Ancient Greek stülos meaning a pillar), is a pillar-like stalk through which pollen tubes grow to reach the ovary. Some flowers such as Tulipa do not have a distinct style, and the stigma sits directly on the ovary. The style is a hollow tube in some plants such as lilies, or has transmitting tissue through which the pollen tubes grow.[8]
- The stigma (from Ancient Greek στίγμα, stigma meaning mark, or puncture), is usually found at the tip of the style, the portion of the carpel(s) that receives pollen (male gametophytes). It is commonly sticky or feathery to capture pollen.
The word "pistil" comes from Latin pistillum meaning pestle.
Carpels
The pistils of a flower are considered to be composed of carpels. A carpel is a theoretical construct interpreted as modified leaves bearing structures called ovules, inside which the egg cells ultimately form. A pistil may consist of one carpel, with its ovary, style and stigma, or several carpels may be joined together with a single ovary, the whole unit called a pistil. The gynoecium may consist of one or more uni-carpellate (with one carpel) pistils, or of one multi-carpellate pistil.
Carpels are thought to be phylogenetically derived from ovule-bearing leaves or leaf homologues (megasporophylls), which evolved to form a closed structure containing the ovules. This structure is typically rolled and fused along the margin.
Although many flowers satisfy the above definition of a carpel, there are also flowers that do not have carpels according to this definition because in these flowers the ovule(s), although enclosed, are borne directly on the shoot apex, and only later become enclosed by the carpel.[5][9] Different remedies have been suggested for this problem. An easy remedy that applies to most cases is to redefine the carpel as an appendage that encloses ovule(s) and may or may not bear them.[6][7][10]


Types of gynoecia
If a gynoecium has a single carpel, it is called monocarpous. If a gynoecium has multiple, distinct (free, unfused) carpels, it is apocarpous. If a gynoecium has multiple carpels "fused" into a single structure, it is syncarpous. A syncarpous gynoecium can sometimes appear very much like a monocarpous gynoecium.
| Gynoecium composition | Carpel terminology |
Pistil terminology | Examples |
|---|---|---|---|
| Single carpel | Monocarpous (unicarpellate) gynoecium | A pistil (simple) | Avocado (Persea sp.), most legumes (Fabaceae) |
| Multiple distinct (unfused) carpels | Apocarpous (choricarpous) gynoecium | Pistils (simple) | Strawberry (Fragaria sp.), Buttercup (Ranunculus sp.) |
| Multiple connate
("fused") carpels |
Syncarpous gynoecium | A pistil (compound) | Tulip (Tulipa sp.), most flowers |
The degree of connation ("fusion") in a syncarpous gynoecium can vary. The carpels may be "fused" only at their bases, but retain separate styles and stigmas. The carpels may be "fused" entirely, except for retaining separate stigmas. Sometimes (e.g., Apocynaceae) carpels are fused by their styles or stigmas but possess distinct ovaries. In a syncarpous gynoecium, the "fused" ovaries of the constituent carpels may be referred to collectively as a single compound ovary. It can be a challenge to determine how many carpels fused to form a syncarpous gynoecium. If the styles and stigmas are distinct, they can usually be counted to determine the number of carpels. Within the compound ovary, the carpels may have distinct locules divided by walls called septa. If a syncarpous gynoecium has a single style and stigma and a single locule in the ovary, it may be necessary to examine how the ovules are attached. Each carpel will usually have a distinct line of placentation where the ovules are attached.
Pistil development
Pistils begin as small primordia on a floral apical meristem, forming later than, and closer to the (floral) apex than sepal, petal and stamen primordia. Morphological and molecular studies of pistil ontogeny reveal that carpels are most likely homologous to leaves.
A carpel has a similar function to a megasporophyll, but typically includes a stigma, and is fused, with ovules enclosed in the enlarged lower portion, the ovary.[11]
In some basal angiosperm lineages, Degeneriaceae and Winteraceae, a carpel begins as a shallow cup where the ovules develop with laminar placentation, on the upper surface of the carpel. The carpel eventually forms a folded, leaf-like structure, not fully sealed at its margins. No style exists, but a broad stigmatic crest along the margin allows pollen tubes access along the surface and between hairs at the margins.[11]
Two kinds of fusion have been distinguished: postgenital fusion that can be observed during the development of flowers, and congenital fusion that cannot be observed i.e., fusions that occurred during phylogeny. But it is very difficult to distinguish fusion and non-fusion processes in the evolution of flowering plants. Some processes that have been considered congenital (phylogenetic) fusions appear to be non-fusion processes such as, for example, the de novo formation of intercalary growth in a ring zone at or below the base of primordia.[12][13][10] Therefore, "it is now increasingly acknowledged that the term 'fusion,' as applied to phylogeny (as in 'congenital fusion') is ill-advised."[14]
Gynoecium position

Basal angiosperm groups tend to have carpels arranged spirally around a conical or dome-shaped receptacle. In later lineages, carpels tend to be in whorls.
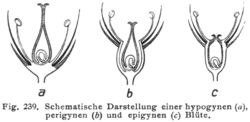
The relationship of the other flower parts to the gynoecium can be an important systematic and taxonomic character. In some flowers, the stamens, petals, and sepals are often said to be "fused" into a "floral tube" or hypanthium. However, as Leins & Erbar (2010) pointed out, "the classical view that the wall of the inferior ovary results from the "congenital" fusion of dorsal carpel flanks and the floral axis does not correspond to the ontogenetic processes that can actually be observed. All that can be seen is an intercalary growth in a broad circular zone that changes the shape of the floral axis (receptacle)."[10] And what happened during evolution is not a phylogenetic fusion but the formation of a unitary intercalary meristem. Evolutionary developmental biology investigates such developmental processes that arise or change during evolution.
If the hypanthium is absent, the flower is hypogynous, and the stamens, petals, and sepals are all attached to the receptacle below the gynoecium. Hypogynous flowers are often referred to as having a superior ovary. This is the typical arrangement in most flowers.
If the hypanthium is present up to the base of the style(s), the flower is epigynous. In an epigynous flower, the stamens, petals, and sepals are attached to the hypanthium at the top of the ovary or, occasionally, the hypanthium may extend beyond the top of the ovary. Epigynous flowers are often referred to as having an inferior ovary. Plant families with epigynous flowers include orchids, asters, and evening primroses.
Between these two extremes are perigynous flowers, in which a hypanthium is present, but is either free from the gynoecium (in which case it may appear to be a cup or tube surrounding the gynoecium) or connected partly to the gynoecium (with the stamens, petals, and sepals attached to the hypanthium part of the way up the ovary). Perigynous flowers are often referred to as having a half-inferior ovary (or, sometimes, partially inferior or half-superior). This arrangement is particularly frequent in the rose family and saxifrages.
Occasionally, the gynoecium is born on a stalk, called the gynophore, as in Isomeris arborea.
Placentation
Within the ovary, each ovule is born by a placenta or arises as a continuation of the floral apex. The placentas often occur in distinct lines called lines of placentation. In monocarpous or apocarpous gynoecia, there is typically a single line of placentation in each ovary. In syncarpous gynoecia, the lines of placentation can be regularly spaced along the wall of the ovary (parietal placentation), or near the center of the ovary. In the latter case, separate terms are used depending on whether or not the ovary is divided into separate locules. If the ovary is divided, with the ovules born on a line of placentation at the inner angle of each locule, this is axile placentation. An ovary with free central placentation, on the other hand, consists of a single compartment without septae and the ovules are attached to a central column that arises directly from the floral apex (axis). In some cases a single ovule is attached to the bottom or top of the locule (basal or apical placentation, respectively).
The ovule
In flowering plants, the ovule (from Latin ovulum meaning small egg) is a complex structure born inside ovaries. The ovule initially consists of a stalked, integumented megasporangium (also called the nucellus). Typically, one cell in the megasporangium undergoes meiosis resulting in one to four megaspores. These develop into a megagametophyte (often called the embryo sac) within the ovule. The megagametophyte typically develops a small number of cells, including two special cells, an egg cell and a binucleate central cell, which are the gametes involved in double fertilization. The central cell, once fertilized by a sperm cell from the pollen becomes the first cell of the endosperm, and the egg cell once fertilized become the zygote that develops into the embryo. The gap in the integuments through which the pollen tube enters to deliver sperm to the egg is called the micropyle. The stalk attaching the ovule to the placenta is called the funiculus.
Role of the stigma and style


Stigmas can vary from long and slender to globe-shaped to feathery. The stigma is the receptive tip of the carpel(s), which receives pollen at pollination and on which the pollen grain germinates. The stigma is adapted to catch and trap pollen, either by combining pollen of visiting insects or by various hairs, flaps, or sculpturings.[15]
The style and stigma of the flower are involved in most types of self incompatibility reactions. Self-incompatibility, if present, prevents fertilization by pollen from the same plant or from genetically similar plants, and ensures outcrossing.
See also
References
- ↑ Judd, W.S.; Campbell, C.S.; Kellogg, E.A.; Stevens, P.F. & Donoghue, M.J. (2007). Plant Systematics: A Phylogenetic Approach (3rd ed.). Sunderland, MA: Sinauer Associates, Inc. ISBN 978-0-87893-407-2.
- ↑ Sattler, R. (1974). "A new approach to gynoecial morphology". Phytomorphology 24: 22–34.
- ↑ Macdonald, A.D. & Sattler, R. (1973). "Floral development of Myrica gale and the controversy over floral theories". Canadian Journal of Botany 51: 1965–1975. doi:10.1139/b73-251.
- ↑ Sattler, R. (1973). Organogenesis of Flowers : a Photographic Text-Atlas. University of Toronto Press. ISBN 978-0-8020-1864-9.
- ↑ 5.0 5.1 Sattler, R. & Lacroix, C. (1988). "Development and evolution of basal cauline placentation: Basella rubra". American Journal of Botany 75: 918–927. doi:10.2307/2444012.
- ↑ 6.0 6.1 Sattler, R. & Perlin, L. (1982). "Floral development of Bougainvillea spectabilis Willd., Boerhaavia diffusa L. and Mirabilis jalapa L. (Nyctaginaceae)". Botanical Journal of the Linnean Society: 161–182. doi:10.1111/j.1095-8339.1982.tb00532.x.
- ↑ 7.0 7.1 Greyson 1994, p. 130.
- ↑ Esau, K. (1965). Plant Anatomy (2nd ed.). New York: John Wiley & Sons. OCLC 263092258.
- ↑ D'Arcy, W.G.; Keating, R.C. (1996). The Anther: Form, Function, and Phylogeny. Cambridge University Press. ISBN 9780521480635.
- ↑ 10.0 10.1 10.2 Leins, P. & Erbar, C. (2010). Flower and Fruit. Stuttgart: Schweizerbart Science Publishers. ISBN 978-3-510-65261-7.
- ↑ 11.0 11.1 Gifford, E.M. & Foster, A.S. (1989). Morphology and Evolution of Vascular Plants (3rd ed.). New York: W.H. Freeman & Co. ISBN 978-0-7167-1946-5.
- ↑ Sattler, R. (1978). "'Fusion' and 'continuity' in floral morphology". Notes of the Royal Botanic Garden, Edinburgh 36: 397–405.
- ↑ Greyson 1994, p. 67–69, 142–145.
- ↑ Greyson 1994, p. 142.
- ↑ Blackmore, Stephen & Toothill, Elizabeth (1984). The Penguin Dictionary of Botany. Penguin Books. ISBN 978-0-14-051126-0.
Bibliography
-
 This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press.
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press. - Greyson, R.I. (1994). The Development of Flowers. Oxford University Press. ISBN 978-0-19-506688-3.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||