Greater short-nosed fruit bat
| Greater short-nosed fruit bat | |
|---|---|
_Photograph_By_Shantanu_Kuveskar.jpg) | |
| Hanging Over Foliage Palm In (Mangaon, Maharashtra, India) | |
| Conservation status | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Chiroptera |
| Family: | Pteropodidae |
| Genus: | Cynopterus |
| Species: | C. sphinx |
| Binomial name | |
| Cynopterus sphinx (Vahl, 1797) | |
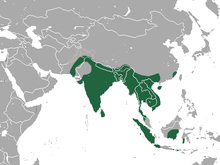 | |
| Greater short-nosed fruit bat range | |
The greater short-nosed fruit bat or short-nosed Indian fruit bat (Cynopterus sphinx) is a species of megabat in the Pteropodidae family. It is found in South and Southeast Asia, where it is known as තල වවුලා (thala wawulaa) by Sinhalese people in Sri Lanka.[1]
Description
These bats have a relatively long snout. Their upper parts are brown to grey-brown with paler under parts. The fur is very fine and silky. The ears and wing bones of C. sphinx are edged in white. Lower cheek teeth rounded without accessory cusps. The wing span of the adult is about 48 cm. Juveniles are lighter than adults. Average forearm length 70.2mm (64-79mm).[2]
Habitat
It is found in Pakistan, Bangladesh, Bhutan, Cambodia, China, India, Indonesia, Laos, Malaysia, Myanmar, Philippines, Sri Lanka, Thailand, and Vietnam. C. sphinx is common in tropical forests and areas where fruit crops are cultivated. They can also be found in grassland and mangrove forests. They typically nest high in palm trees. The bats chew the fronds of the palms to construct fairly simple tents. These bats are also known to construct tents by closely interweaving the leaves and twigs of creeping vines which cover buildings, but such nests are constructed only when palms are not available.
Behaviour and breeding
The species is gregarious, and typically roosts in same sex groups of 8-9 individuals. The sexes remain separate until the mating season, when group size increases. They are polygynous and it is usual for 6-10 males and 10-15 females to share palm frond tents during the breeding season.[3] C. sphinx is the only non-primate species to show fellatio, which enhances copulation time in the species. Copulation by males is dorsoventral and the females lick the shaft or the base of the male's penis, but not the glans which has already penetrated the vagina. While the females do this, the penis is not withdrawn and research has shown a positive relationship between length of the time that the penis is licked and the duration of copulation. Post copulation genital grooming has also been observed.[4] Males stay with females for some time after mating, but later return to same sex groups.
The adult sex ratio is very female biased. Researchers attribute this to the relatively rapid maturation of females compared to males. In Central India, C. sphinx breeds twice per year. Females produce a single young at a time. Each half of the bicornate uterus functions during alternate breeding cycles. The first pregnancy cycle occurs from October through February/March. Mating occurs immediately postpartum, and a second offspring is born in July. Gestation period is about 3–5 months. In 72% of bats, the first pregnancy occurs in the right horn of the uterus. The corpus luteum in the right ovary persists for some time after the pregnancy and prevents ovulation from occurring in the right ovary during the second breeding cycle. This creates the pattern of alternate functioning of the two horns of the uterus. However, the corpus luteum in the left ovary does not persist until the beginning of the next breeding cycle. As yet, no reason has been found for the dominance of the right horn during the first breeding cycle.[5][6] Newborn bats weigh about 13.5 g and have a wingspan of 24 cm. By the time of weaning at 4 weeks of age, young bats weigh 25 g and have wings spanning 36 cm. Female short-nosed fruit bats reach sexual maturity at 5–6 months of age, but males are not capable of breeding until they are a year old.[7]
These bats are frugivorous, locate their preferred food items by scent. They have been described as voracious feeders, eating more than their body weight in food in one sitting. Some preferred fruits include ripe guava, banana, chikoo, dates and lychees.
Short-nosed fruit bats inflict serious damage on many fruit crops, and are considered pests. In addition, these bats are possible vectors for Japanese encephalitis, which is serious disease in humans.[8] These bats are important dispersers of date palm seeds, and pollinate many night blooming flowers.
Notes
- ↑ Bates, P; Bumrungsri, Molur S. & Srinivasulu, C. (2008). "IUCN entry for C. sphinx". IUCN Red List of Threatened Species. Retrieved 2009-10-28.
- ↑ Bates, P.J.J; D.A. Harrison (1997). Bats of the Indian Subcontinent. Harrison Zoological Museum. p. 258. ISBN 0951731319.
- ↑ Balasingh, J; J. Suthakar-Isaac, S., and R. Subbaraj (1993). "Tent roosting by the frugivorous bat Cynopterus sphinx in southern India". Current Science 65 (5): 418.
- ↑ 4.0 4.1 Tan, Min; Gareth Jones; Guangjian Zhu; Jianping Ye; Tiyu Hong; Shanyi Zhou; Shuyi Zhang; Libiao Zhang (October 28, 2009). Hosken, David, ed. "Fellatio by Fruit Bats Prolongs Copulation Time". PLoS ONE 4 (10): e7595. Bibcode:2009PLoSO...4.7595T. doi:10.1371/journal.pone.0007595. PMC 2762080. PMID 19862320. Retrieved October 28, 2009.
- ↑ Advani, R (1982). "Feeding, foraging and roosting behavior of the fruit eating bats and damage to fruit crops in Rajasthan and Gujarat". Saeugeteirkundliche mitteilungen 30 (1): 46–48.
- ↑ Sandhu, S; A. Gopalakrishna. (1984). "Some observations on the breeding biology of Cynopterus sphinx in central India". Current Science 53 (22): 1189–1192.
- ↑ Krishna, A; C.J. Dominic (1983). "Growth of young and sexual maturity of 3 species of Indian Bats". Journal of Animal Morphology and Physiology 30 (1–2): 162–168.
- ↑ Banjeree, K; Ilkal, M.A., and P.K. Deshmikh. (1984). "Susceptibility of Cynopterus sphinx (frugivorus bat) and Suncus minimus (house shrew) to Japanese encephalitis virus". Indian Journal of Medical Research 79 (1): 8–12.
References
- Nowak, Ronald. 1991 Walker's Mammals of the World, Fifth edition. Johns Hopkins University Press, Baltimore and London.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

