Gramicidin S
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
| Gramicidin S | |
| Clinical data | |
| Topical | |
| Identifiers | |
|
113-73-5 | |
| PubChem | CID 73357 |
| ChemSpider |
66085 |
| ChEBI |
CHEBI:5530 |
| ChEMBL |
CHEMBL373496 |
| NIAID ChemDB | 002002 |
| Chemical data | |
| Formula | C60H92N12O10 |
| 1140.7059 g/mol | |
|
SMILES
| |
| |
| | |
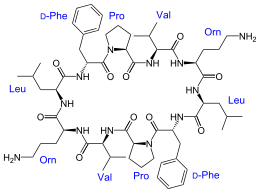
Gramicidin S or Gramicidin Soviet[1] is an antibiotic effective against some Gram positive and Gram negative bacteria as well as some fungi. It is a derivative of gramicidin, produced by the Gram positive bacterium Bacillus brevis. Gramicidin S is a cyclodecapeptide, constructed as two identical pentapeptides joined head to tail, formally written as cyclo(-Val-Orn-Leu-D-Phe-Pro-)2. That is to say, it forms a ring structure composed of five different amino acids, each one used twice within the structure.[2] Another interesting point is that it utilizes two amino acids uncommon in peptides: ornithine as well as the atypical stereoisomer of phenylalanine. It is synthesized by gramicidin S synthetase.[3]
History
Gramicidin S was discovered by Russian microbiologist Georgyi Frantsevitch Gause and his wife Maria Brazhnikova in 1942. Within the year Gramicidin S was being used in Soviet military hospitals to treat infection and eventually found usage at the front lines of combat by 1946.[4] Gause was awarded the Stalin Prize for Medicine for his discovery in 1946. In 1944, Gramicidin S was sent by the Ministry of Health of the USSR to Great Britain via the International Red Cross in a collaborative effort to establish the exact structure. English chemist Richard Synge proved that the compound was an original antibiotic and a polypeptide using paper chromatography.[5] He would later go on to receive the Nobel Prize for his work in chromatography. The crystal structure was finally established by Dorothy Hodgkin and Gerhard Schmidt; Margaret Thatcher who worked for a term in 1947 with Gerhard Schmidt on the antibiotic Gramicidin S, as an undergraduate research project. The importance of Gramicidin S and antibiotic research in general was so great that Gause was not persecuted during the period of Lysenkoism in the USSR, while many of his colleagues were being executed. Indeed, it was his need for developing new strains to mass-produce antibiotics that allowed politically sanctioned collaborations with geneticists like Joseph Rapoport and Alexander Malinovsky, who would both actively participate in the downfall of Lysenkoism.[4]
Structure and pharmacological effect
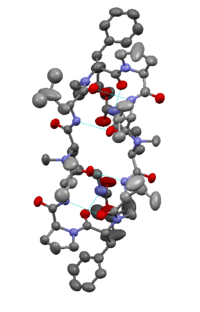
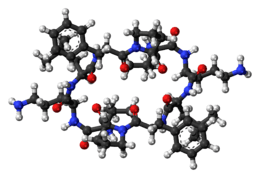
Structurally, Gramicidin S differs from Gramicidin D, which is a linear peptide and forms a beta helix in cellular membranes. The mode of action is not entirely agreed upon, but it is generally accepted that it is the disruption of the barrier properties of cellular membranes which causes cell death. Recent research reveals that Gramicidin S interacts more so with anionic membranes (such as those of bacteria) vs. zwitterionic membranes and more fluid membranes.[7] It has a molecular mass of ca. 1,140 and is a solid, usually encapsulated in two-percent sterile spirit solution. In vitro assays show it has a MIC of 5-15 μg/mL.[8]
Use
Gramicidin S has historically been employed as a topical antibiotic for the treatment of infections from superficial wounds. It exhibits strong antibiotic activity against a broad spectrum of Gram negative and Gram-positive bacteria and against several pathogenic fungi. Like Gramicidin D, Gramicidin S causes hemolysis at low concentrations, thus is not an effective drug for the treatment of systemic infections. Additionally, Gramicidin S has been employed as a spermicide and therapeutic for genital ulcers caused by sexually transmitted disease.[9]
References
- ↑ Gause, G. F.; Brazhnikova, M. G. (1944), "Gramicidin S and its use in the Treatment of Infected Wounds", Nature 154 (3918): 703–703, doi:10.1038/154703a0.
- ↑ Llamas-Saiz, Antonio; Grotenbreg, GM; Overhand, M; Van Raaij, MJ (2007), "Double-stranded helical twisted b-sheet channels in crystals of gramicidin S grown in the presence of trifluoroacetic and hydrochloric acids", Acta Crystallographica Section D D63 (Pt 3): 401–407, doi:10.1107/S0907444906056435, PMID 17327677
- ↑ Conti, E; Stachelhaus, T; Marahiel, MA; Brick, P (1997), "Structural basis for the activation of phenylalanine in the non-ribosomal biosynthesis of gramicidin S", The EMBO Journal 16 (14): 4174–4183, doi:10.1093/emboj/16.14.4174, PMC 1170043, PMID 9250661
- ↑ 4.0 4.1 Gall, YM; Konashev, MB (2001), "The discovery of Gramicidin S: the Intellectual Transformation of G.F. Gause from Biologist to Researcher of Antibiotics and on its Meaning for the Fate of Russian Genetics", Hist. Phil. Life Sci. 23 (1): 137–50, PMID 12212443
- ↑ R.L.M. Synge - Britannica Online Encyclopedia
- ↑ Yamada, Keiichi; Unno, Masafumi; Kobayashi, Kyoko; Oku, Hiroyuki; Yamamura, Hatsuo; Araki, Shuki; Matsumoto, Hideyuki; Katakai, Ryoichi; Kawai, Masao (2002), "Stereochemistry of Protected Ornithine Side Chains of Gramicidin S Derivatives: X-ray Crystal Structure of the Bis-Boc-tetra-N-methyl Derivative of Gramicidin S", Journal of the American Chemical Society 124 (43): 12684–12688, doi:10.1021/ja020307t, PMID 12392415
- ↑ Prenner, E. J.; Kiricsi, M; Jelokhani-Niaraki, M; Lewis, RN; Hodges, RS; McElhaney, RN (2004), "Structure-Activity Relationships of Diastereomeric Lysine Ring Size Analogs of the Antimicrobial Peptide Gramicidin S: MECHANISM OF ACTION AND DISCRIMINATION BETWEEN BACTERIAL AND ANIMAL CELL MEMBRANES", Journal of Biological Chemistry 280 (3): 2002–2011, doi:10.1074/jbc.M406509200, PMID 15542606.
- ↑ Xiao, Jingbo; Weisblum, Bernard; Wipf, Peter (2005), "Electrostatic versus Steric Effects in Peptidomimicry: Synthesis and Secondary Structure Analysis of Gramicidin S Analogues with (E)-Alkene Peptide Isosteres", Journal of the American Chemical Society 127 (16): 5742–5743, doi:10.1021/ja051002s, PMID 15839644.
- ↑ Krylov YuF (1993) Compendium of medicinal products of Russia. Inpharmchem Press, Moscow, p 343 [in Russian]
| ||||||||||||||||||||||||||||||||||||||||