Grain growth
Grain growth is the increase in size of grains (crystallites) in a material at high temperature. This occurs when recovery and recrystallisation are complete and further reduction in the internal energy can only be achieved by reducing the total area of grain boundary. The term is commonly used in metallurgy but is also used in reference to ceramics and minerals.
Importance of grain growth
Most materials exhibit the Hall–Petch effect at room-temperature and so display a higher yield stress when the grain size is reduced. At high temperatures the opposite is true since the open, disordered nature of grain boundaries means that vacancies can diffuse more rapidly down boundaries leading to more rapid Coble creep. Since boundaries are regions of high energy they make excellent sites for the nucleation of precipitates and other second-phases e.g. Mg–Si–Cu phases in some aluminium alloys or martensite platlets in steel. Depending on the second phase in question this may have positive or negative effects.
Rules of grain growth
Grain growth has long been studied primarily by the examination of sectioned, polished and etched samples under the optical microscope. Although such methods enabled the collection of a great deal of empirical evidence, particularly with regard to factors such as temperature or composition, the lack of crystallographic information limited the development of an understanding of the fundamental physics. Nevertheless, the following became well-established features of grain growth:
- Grain growth occurs by the movement of grain boundaries and not by coalescence (i.e. like water droplets)
- Boundary movement is discontinuous and the direction of motion may change suddenly.
- One grain may grow into another grain whilst being consumed from the other side
- The rate of consumption often increases when the grain is nearly consumed
- A curved boundary typically migrates towards its centre of curvature
- When grain boundaries in a single phase meet at angles other than 120 degrees, the grain included by the more acute angle will be consumed so that the angles approach 120 degrees.
Driving force
The boundary between one grain and its neighbour (grain boundary) is a defect in the crystal structure and so it is associated with a certain amount of energy. As a result, there is a thermodynamic driving force for the total area of boundary to be reduced. If the grain size increases, accompanied by a reduction in the actual number of grains per volume, then the total area of grain boundary will be reduced.
The local velocity of a grain boundary at any point is proportional to the local curvature of the grain boundary, i.e.:
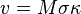 ,
,
where 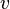 is the velocity of grain boundary,
is the velocity of grain boundary, 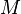 is grain boundary mobility (generally depends on orientation of two grains),
is grain boundary mobility (generally depends on orientation of two grains), 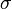 is the grain boundary energy and
is the grain boundary energy and 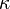 is the sum of the two principal surface curvatures. For example, shrinkage velocity of a spherical grain embedded inside another grain is
is the sum of the two principal surface curvatures. For example, shrinkage velocity of a spherical grain embedded inside another grain is
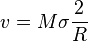 ,
,
where 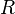 is radius of the sphere. This driving pressure is very similar in nature to the Laplace pressure that occurs in foams.
is radius of the sphere. This driving pressure is very similar in nature to the Laplace pressure that occurs in foams.
In comparison to phase transformations the energy available to drive grain growth is very low and so it tends to occur at much slower rates and is easily slowed by the presence of second phase particles or solute atoms in the structure.
Ideal grain growth

Ideal grain growth is a special case of normal grain growth where boundary motion is driven only by local curvature of the grain boundary. It results in the reduction of the total amount of grain boundary surface area i.e. total energy of the system. Additional contributions to the driving force by e.g. elastic strains or temperature gradients are neglected. If it holds that the rate of growth is proportional to the driving force and that the driving force is proportional to the total amount of grain boundary energy, then it can be shown that the time t required to reach a given grain size is approximated by the equation
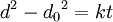
where d0 is the initial grain size, d is the final grain size and k is a temperature dependent constant given by an exponential law:
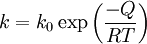
where k0 is a constant, T is the absolute temperature and Q is the activation energy for boundary mobility. Theoretically, the activation energy for boundary mobility should equal that for self-diffusion but this is often found not to be the case.
In general these equations are found to hold for ultra-high purity materials but rapidly fail when even tiny concentrations of solute are introduced.
Normal vs abnormal
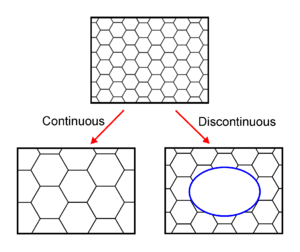
In common with recovery and recrystallisation, growth phenomena can be separated into continuous and discontinuous mechanisms. In the former the microstructure evolves from state A to B (in this case the grains get larger) in a uniform manner. In the latter, the changes occur heterogeneously and specific transformed and untransformed regions may be identified. Abnormal or discontinuous grain growth is characterised by a subset of grains growing at a high rate and at the expense of their neighbours and tends to result in a microstructure dominated by a few very large grains. In order for this to occur the subset of grains must possess some advantage over their competitors such as a high grain boundary energy, locally high grain boundary mobility, favourable texture or lower local second-phase particle density.
Factors hindering growth
If there are additional factors preventing boundary movement, such as Zener pinning by particles, then the grain size may be restricted to a much lower value than might otherwise be expected. This is an important industrial mechanism in preventing the softening of materials at high temperature.
Inhibition
Certain materials especially refractories which are processed at high temperatures end up with excessively large grain size and poor mechanical properties at room temperature. To mitigate this problem in a common sintering procedure, a variety of dopants are often used to inhibit grain growth.
References
- F. J. Humphreys and M. Hatherly (1995); Recrystallization and related annealing phenomena, Elsevier