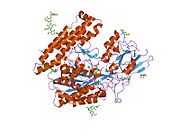
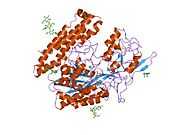
Glutamate carboxypeptidase II
| glutamate carboxypeptidase II | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Reaction Scheme of NAAG Degradation by GCPII: GCPII + NAAG → GCPII-NAAG complex → Glutamate + NAA | |||||||||
| Identifiers | |||||||||
| EC number | 3.4.17.21 | ||||||||
| CAS number | 111070-04-3 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
Glutamate carboxypeptidase II (GCPII), also known as N-acetyl-L-aspartyl-L-glutamate peptidase I (NAALADase I), NAAG peptidase, or prostate-specific membrane antigen (PSMA) is an enzyme that in humans is encoded by the FOLH1 (folate hydrolase 1) gene.[1] Human GCPII contains 750 amino acids and weighs approximately 84 kDa.[2]
GCPII is a zinc metalloenzyme that resides in membranes. Most of the enzyme resides in the extracellular space. GCPII is a class II membrane glycoprotein. It catalyzes the hydrolysis of N-acetylaspartylglutamate (NAAG) to glutamate and N-acetylaspartate (NAA) according to the reaction scheme to the right.[3][4]
Neuroscientists primarily use the term NAALADase in their studies, while those studying folate metabolism use folate hydrolase, and those studying prostate cancer or oncology, PSMA. All of which refer to the same protein glutamate carboxypeptidase II.
Discovery
GCPII is expressed in many tissues, including the prostate, kidney, the small intestine, and the central and peripheral nervous system.[2]
Indeed the initial cloning of the cDNA encoding the gene expressing PSMA was accomplished with RNA from a prostate tumor cell line, LNCaP.[5] PSMA shares homology with the transferrin receptor and undergoes endcytosis but the ligand for inducing internalization has not been identified.[6] It was found that PSMA was the same as the membrane protein in the small intestine responsible for removal of gamma-linked glutamates from polygammaglutamate folate. This enables the freeing of folic acid, which then can be transported into the body for use as a vitamin. This resulted in the cloned genomic designation of PSMA as FOLH1 for folate hydrolase.[7]
PSMA(FOLH1)+ folate polygammaglutamate(n 1-7)---> PSMA (FOLH1) + folate(poly)gammaglutamate(n-1) + glutamate continuing until releasing folate.
Structure
The three domains of the extracellular portion of GCPII –the protease, apical and C-terminal domains- collaborate in substrate recognition.[4] The protease domain is a central seven-stranded mixed β-sheet. The β-sheet is flanked by 10 α-helices. The apical domain is located between the first and the second strands of the central β-sheet of the protease domain. The apical domain creates a pocket that facilitates substrate binding. The C-terminal domain is an Up-Down-Up-Down four-helix bundle.
The central pocket is approximately 2 nanometres in depth and opens from the extracellular space to the active site.[4] This active site contains two zinc ions. During inhibition, each acts as a ligand to an oxygen in 2-PMPA or phosphate.
There is also one calcium ion coordinated in GCPII, far from the active site. It has been proposed that calcium holds together the protease and apical domains.[4]
In addition, ten glycosylation sites have been identified in human GCPII.[2] Glycosylation far from the catalytic domain still affects the ability of GCPII to hydrolyze NAAG.[2]
Enzyme kinetics
The hydrolysis of NAAG by GCPII obeys Michaelis-Menten kinetics calculated the binding constant (Km) for NAAG as approximately 130 nM and the turnover constant (kcat) as approximately 4 s−1.[4] The apparent second-order rate constant is approximately 3 x 107 (M·s)−1.
Role in prostate cancer
It was found that there were multiple potential start sites for PSMA as well as multiple alternative splice forms that vary in the type of membrane protein formed or having a cytosolic location and each form probably varies regarding caboxypeptidase activity given the restriction for enzymatic activity for PSMA.[2][8] PSMA is strongly expressed in the human prostate, being a hundredfold greater than the expression in most other tissues. In cancer, it is upregulated in expression and has been called the second-most-upregulated gene in prostate cancer, with increase of 8- to 12-fold over the noncancerous prostate.[9] Because of this high expression, it is being developed as a target for therapy and imaging.[10] In human prostate cancer, the higher expressing tumors are associated with quicker time to progression and a greater percentage of patients suffering relapse.[11][12] PSMA is the target of an approved imaging agent for prostate cancer, capromabpentide, PROSTASCINT. Second-generation antibodies and low-molecular-weight ligands for imaging and therapy are being developed.[10][13][14][15][16][17][18]
In addition to the expression in the human prostate and prostate cancer, PSMA is also found to be highly expressed in tumor neovasculature but not normal vasculature of all types of solid tumors as kidney, breast, colon, etc.[19] In terms of imaging, no non-tumor site such as normal kidney, small intestine, or CNS has been imaged using second-generation antibody imaging agents, while sites even in bone are being detected with better sensitivity than with technetium scans, and the tumors expressing PSMA in their neovasculature are also being imaged.[14] Low-molecular-weight ligands exhibit different binding with imaging seen in the kidney of the mouse, however mouse has much higher levels of PSMA in kidney and brain than the human. In the mouse, it was only the normal kidney and prostate tumors that were imaged, and not other tissues, not even CNS suggesting the imprortance of the blood–brain barrier.[13] In kidney, it is a subset of tubules that contain PSMA. Thus, imaging studies will have to be performed in humans with the low-molecular-weight ligands to define their potential for imaging and targeting. Still, in terms of potential toxicities, knockout animals were normal on most tests, which reduces somewhat concerns about toxicity in targeting PSMA.[20] In the CNS, PSMA is present only in a sub-set of glial cells, again suggesting that toxin targeting would likely have minimal toxicity to the host even if the blood–brain barrier were not intact.[21]
Neurotransmitter degradation
For those studying neural based diseases, NAAG is one of the three most prevalent neurotransmitters found in the central nervous system[22] and when it catylizes the reaction to produce glutamate it is also producing another neurotransmitter.[4] Glutamate is a common and abundant excitatory neurotransmitter in the central nervous system; however, if there is too much glutamate transmission, this can kill or at least damage neurons and has been implicated in many neurological diseases and disorders[22] therefore the balance that NAAG peptidase contributes to is quite important.
Potential therapeutic applications
Function in the brain
GCPII has been shown to both indirectly and directly increase the concentration of glutamate in the extracellular space.[22] GCPII directly cleaves NAAG into NAA and glutamate.[3][4] NAAG has been shown, in high concentration, to indirectly inhibit the release of neutrotransmitters, such as GABA and glutamate. It does this through interaction with and activation of presynaptic group II mGluRs.[22] Thus, in the presence of NAAG peptidase, the concentration of NAAG is kept in check, and glutamate and GABA, among other neurotransmitters, are not inhibited.
Researchers have been able to show that effective and selective GCPII inhibitors are able to decrease the brain's levels of glutamate and even provide protection from apoptosis or degradation of brain neurons in many animal models of stroke, amyotrophic lateral sclerosis, and neuropathic pain.[4] This inhibition of these NAAG peptidases, sometimes referred to as NPs, are thought to provide this protection from apoptosis or degradation of brain neurons by elevating the concentrations of NAAG within the synapse of neurons.[22] NAAG then reduces the release of glutamate while stimulating the release of some trophic factors from the glia cells in the central nervous system, resulting in the protection from apoptosis or degradation of brain neurons.[22] It is important to note, however, that these NP inhibitors do not seem to have any effect on normal glutamate function.[22] The NP inhibition is able to improve the naturally occurring regulation instead of activating or inhibiting receptors that would disrupt this process.[22] Research has also shown that small-molecule-based NP inhibitors are beneficial in animal models that are relevant to neurodegenerative diseases.[22] Some specific applications of this research include neuropathic and inflammatory pain, traumatic brain injury, ischemic stroke, schizophrenia, diabetic neuropathy, amyotrophic lateral sclerosis, as well as drug addiction.[22] Previous research has found that drugs that are able to reduce glutamate transmission can relieve the neuropathic pain, although the resultant side-effects have limited a great deal of their clinical applications.[23] Therefore, it appears that, since GCPII is exclusively recruited for the purpose of providing a glutamate source in hyperglutamatergic and excitotoxic conditions, this could be an alternative to avert these side-effects.[23] More research findings have shown that the hydrolysis of NAAG is disrupted in schizophrenia, and they have shown that specific anatomical regions of the brain may even show discrete abnormalities in the GCP II synthesis, so NPs may also be therapeutic for patients suffering with schizophrenia.[24] One major hurdle with using many of the potent GCPII inhibitors that have been prepared to date are typically highly polar compounds, which causes problems because they do not then penetrate the blood–brain barrier easily.[25]
Potential uses of NAAG peptidase inhibitors
Glutamate is the “primary excitatory neurotransmitter in the human nervous system”,[22] participating in a multitude of brain functions. Over-stimulation and -activation of glutamate receptors as well as “disturbances in the cellular mechanisms that protect against the adverse consequences of physiological glutamate receptor activation”[25] have been known to cause neuron damage and death, which have been associated with multiple neurological diseases.[22]
Due to the range of glutamate function and presence, it has been difficult to create glutamatergic drugs that do not negatively affect other necessary functions and cause unwanted side-effects.[26] NAAG peptidase inhibition has offered the possibility for specific drug targeting.
Specific inhibitors
Since its promise for possible neurological disease therapy and specific drug targeting, NAAG peptidase inhibitors have been widely created and studied. A few small molecule examples are those that follow:[22]
- 2-PMPA and analogues
- Thiol and indole thiol derivatives
- Hydroxamate derivatives
- Conformationally constricted dipeptide mimetics
- PBDA- and urea-based inhibitors.
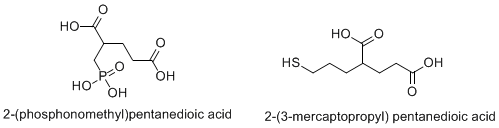
Other potential therapeutic applications
Neuropathic and inflammatory pain
Pain cause by injury to CNS or PNS has been associated with increase glutamate concentration. NAAG inhibition reduced glutamate presence and could, thus, diminish pain.[22] (Neale JH et al., 2005). Nagel et al.[26] used the inhibitor 2-PMPA to show the analgesic effect of NAAG peptidase inhibitions. This study followed one by Chen et al.,[27] which showed similar results.[26]
Head injury
Severe head injury (SHI) and traumatic brain injury (TBI) are widespread and have a tremendous impact. “They are the leading cause of death in children and young adults (<25 years) and account for a quarter of all deaths in the five to 15 years age group”.[28] Following initial impact, glutamate levels rise and cause excitotoxic damage in a process that has been well characterized.[22] With its ability to reduce glutamate levels, NAAG inhibition has shown promise in preventing neurological damage associated with SHI and TBI.
Stroke
According to the National Stroke Association,[29] stroke is the third-leading cause of death and the leading cause of adult disability. It is thought that glutamate levels cause underlying ischemic damage during a stroke, and, thus, NAAG inhibition might be able to diminish this damage.[22]
Schizophrenia
Schizophrenia is a mental disorder that affects 1% of people throughout the world.[30] It can be modeled by PCP in laboratory animals, and it has been shown that mGluR agonists have reduced the effects of the drug. NAAG is, such, an mGluR agonist. Thus, inhibition of the enzyme that reduces NAAG concentration, NAAG peptidase, could provide a practical treatment for reduction of schizophrenic symptoms.[22]
Diabetic neuropathy
Diabetes can lead to damaged nerves, causing loss of sensation, pain, or, if autonomic nerves are associated, damage to the circulatory, reproductive, or digestive systems, among others. Over 60% of diabetic patients are said to have some form of neuropathy,[22] however the severity ranges dramatically. Neuropathy not only directly causes harm and damage but also can indirectly lead to such problems as diabetic ulcerations, which in turn can lead to amputations. In fact, over half of all lower limb amputations in the United States are of patients with diabetes.[31]
Through the use of the NAAG peptidase inhibitor 2-PMPA, NAAG cleavage was inhibited and, with it, programmed DRG neuronal cell death in the presence of high glucose levels.[32] The researchers have proposed that the cause of this is NAAG’s agonistic activity at mGluR3. In addition, NAAG also “prevented glucose-induced inhibition of neurite growth” (Berent- Spillson, et al. 2004). Overall, this makes GCPIII inhibition a clear model target for combating diabetic neuropathy.
Drug addiction
Schizophrenia, as previously described, is normally modeled in the laboratory through a PCP animal model. As GCPIII inhibition was shown to possibly limit schizophrenic behavior in this model,[22] this suggests that GCPIII inhibition, thus, reduces the effect of PCP. In addition, the reward action of many drugs (cocaine, PCP, alcohol, nicotine, etc.) have been shown with increasing evidence to be related to glutamate levels, on which NAAG and GCPIII can have some regulatory effect.[22]
In summary, the findings of multiple drug studies to conclude that:[22]
- NAAG/NP system might be involved in neuronal mechanisms regulating cue-induced cocaine craving, the development of cocaine seizure kindling, and management of opioid addiction and alcohol consumptive behaviour. Therefore, NP inhibitors could provide a novel therapy for such conditions.
Other diseases and disorders
NAAG inhibition has also been studied as a treatment against prostate cancer, ALS, and other neurodegenerative diseases such as Parkinson’s disease and Huntington’s disease.[22]
References
- ↑ O'Keefe DS, Su SL, Bacich DJ, Horiguchi Y, Luo Y, Powell CT, Zandvliet D, Russell PJ, Molloy PL, Nowak NJ, Shows TB, Mullins C, Vonder Haar RA, Fair WR, Heston WD (November 1998). "Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene". Biochim. Biophys. Acta 1443 (1–2): 113–27. doi:10.1016/s0167-4781(98)00200-0. PMID 9838072.
- ↑ 2.0 2.1 2.2 2.3 2.4 Barinka C, Sácha P, Sklenár J, Man P, Bezouska K, Slusher BS, Konvalinka J (June 2004). "Identification of the N-glycosylation sites on glutamate carboxypeptidase II necessary for proteolytic activity". Protein Sci. 13 (6): 1627–35. doi:10.1110/ps.04622104. PMC 2279971. PMID 15152093.
- ↑ 3.0 3.1 Rojas C, Frazier ST, Flanary J, Slusher BS (November 2002). "Kinetics and inhibition of glutamate carboxypeptidase II using a microplate assay". Anal. Biochem. 310 (1): 50–4. doi:10.1016/S0003-2697(02)00286-5. PMID 12413472.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Mesters JR, Barinka C, Li W, Tsukamoto T, Majer P, Slusher BS, Konvalinka J, Hilgenfeld R (March 2006). "Structure of glutamate carboxypeptidase II, a drug target in neuronal damage and prostate cancer". EMBO J. 25 (6): 1375–84. doi:10.1038/sj.emboj.7600969. PMC 1422165. PMID 16467855.
- ↑ Israeli RS, Powell CT, Fair WR, Heston WD (January 1993). "Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen". Cancer Res. 53 (2): 227–30. PMID 8417812.
- ↑ Goodman OB, Barwe SP, Ritter B, McPherson PS, Vasko AJ, Keen JH, Nanus DM, Bander NH, Rajasekaran AK (November 2007). "Interaction of prostate specific membrane antigen with clathrin and the adaptor protein complex-2". Int. J. Oncol. 31 (5): 1199–203. doi:10.3892/ijo.31.5.1199. PMID 17912448.
- ↑ Pinto JT, Suffoletto BP, Berzin TM, Qiao CH, Lin S, Tong WP, May F, Mukherjee B, Heston WD (September 1996). "Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells". Clin. Cancer Res. 2 (9): 1445–51. PMID 9816319.
- ↑ O'Keefe DS, Bacich DJ, Heston WDW (2001). "Prostate Specific Membraen Antigen". In Simons JW, Chung LWK, Isaacs WB. Prostate cancer: biology, genetics and the new therapeutics. Totowa, NJ: Humana Press. pp. 307–326. ISBN 0-89603-868-8.
- ↑ O'Keefe DS, Bacich DJ, Heston WD (February 2004). "Comparative analysis of prostate-specific membrane antigen (PSMA) versus a prostate-specific membrane antigen-like gene". Prostate 58 (2): 200–10. doi:10.1002/pros.10319. PMID 14716746.
- ↑ 10.0 10.1 Wang X, Yin L, Rao P, Stein R, Harsch KM, Lee Z, Heston WD (October 2007). "Targeted treatment of prostate cancer". J. Cell. Biochem. 102 (3): 571–9. doi:10.1002/jcb.21491. PMID 17685433.
- ↑ Perner S, Hofer MD, Kim R, Shah RB, Li H, Möller P, Hautmann RE, Gschwend JE, Kuefer R, Rubin MA (May 2007). "Prostate-specific membrane antigen expression as a predictor of prostate cancer progression". Hum. Pathol. 38 (5): 696–701. doi:10.1016/j.humpath.2006.11.012. PMID 17320151.
- ↑ Ross JS, Sheehan CE, Fisher HA, Kaufman RP, Kaur P, Gray K, Webb I, Gray GS, Mosher R, Kallakury BV (December 2003). "Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer". Clin. Cancer Res. 9 (17): 6357–62. PMID 14695135.
- ↑ 13.0 13.1 Foss CA, Mease RC, Fan H, Wang Y, Ravert HT, Dannals RF, Olszewski RT, Heston WD, Kozikowski AP, Pomper MG (June 2005). "Radiolabeled small-molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer". Clin. Cancer Res. 11 (11): 4022–8. doi:10.1158/1078-0432.CCR-04-2690. PMID 15930336.
- ↑ 14.0 14.1 Milowsky MI, Nanus DM, Kostakoglu L, Sheehan CE, Vallabhajosula S, Goldsmith SJ, Ross JS, Bander NH (February 2007). "Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors". J. Clin. Oncol. 25 (5): 540–7. doi:10.1200/JCO.2006.07.8097. PMID 17290063.
- ↑ Kularatne SA, Zhou Z, Yang J, Post CB, Low PS (May 2009). "Design, synthesis, and preclinical evaluation of prostate-specific membrane antigen targeted (99m)Tc-radioimaging agents". Mol. Pharm. 6 (3): 790–800. doi:10.1021/mp9000712. PMID 19361232.
- ↑ Kularatne SA, Venkatesh C, Santhapuram HK, Wang K, Vaitilingam B, Henne WA, Low PS (November 2010). "Synthesis and biological analysis of prostate-specific membrane antigen-targeted anticancer prodrugs". J. Med. Chem. 53 (21): 7767–77. doi:10.1021/jm100729b. PMID 20936874.
- ↑ Thomas M, Kularatne SA, Qi L, Kleindl P, Leamon CP, Hansen MJ, Low PS (September 2009). "Ligand-targeted delivery of small interfering RNAs to malignant cells and tissues". Ann. N. Y. Acad. Sci. 1175: 32–9. doi:10.1111/j.1749-6632.2009.04977.x. PMID 19796075.
- ↑ He W, Kularatne SA, Kalli KR, Prendergast FG, Amato RJ, Klee GG, Hartmann LC, Low PS (October 2008). "Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands". Int. J. Cancer. 123 (8): 1968–73. doi:10.1002/ijc.23717. PMC 2778289. PMID 18661519.
- ↑ Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB (July 1999). "Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature". Cancer Res. 59 (13): 3192–8. PMID 10397265.
- ↑ Bacich DJ, Wozniak KM, Lu XC, O'Keefe DS, Callizot N, Heston WD, Slusher BS (October 2005). "Mice lacking glutamate carboxypeptidase II are protected from peripheral neuropathy and ischemic brain injury". J. Neurochem. 95 (2): 314–23. doi:10.1111/j.1471-4159.2005.03361.x. PMID 16190866.
- ↑ Sácha P, Zámecník J, Barinka C, Hlouchová K, Vícha A, Mlcochová P, Hilgert I, Eckschlager T, Konvalinka J (February 2007). "Expression of glutamate carboxypeptidase II in human brain". Neuroscience 144 (4): 1361–72. doi:10.1016/j.neuroscience.2006.10.022. PMID 17150306.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 22.6 22.7 22.8 22.9 22.10 22.11 22.12 22.13 22.14 22.15 22.16 22.17 22.18 22.19 22.20 22.21 Zhou J, Neale JH, Pomper MG, Kozikowski AP (December 2005). "NAAG peptidase inhibitors and their potential for diagnosis and therapy". Nat Rev Drug Discov 4 (12): 1015–26. doi:10.1038/nrd1903. PMID 16341066.
- ↑ 23.0 23.1 Zhang W, Murakawa Y, Wozniak KM, Slusher B, Sima AA (September 2006). "The preventive and therapeutic effects of GCPII (NAALADase) inhibition on painful and sensory diabetic neuropathy". J. Neurol. Sci. 247 (2): 217–23. doi:10.1016/j.jns.2006.05.052. PMID 16780883.
- ↑ Ghose S, Weickert CS, Colvin SM, Coyle JT, Herman MM, Hyde TM, Kleinman JE (January 2004). "Glutamate carboxypeptidase II gene expression in the human frontal and temporal lobe in schizophrenia". Neuropsychopharmacology 29 (1): 117–25. doi:10.1038/sj.npp.1300304. PMID 14560319.
- ↑ 25.0 25.1 Kozikowski AP, Nan F, Conti P, Zhang J, Ramadan E, Bzdega T, Wroblewska B, Neale JH, Pshenichkin S, Wroblewski JT (February 2001). "Design of remarkably simple, yet potent urea-based inhibitors of glutamate carboxypeptidase II (NAALADase)". J. Med. Chem. 44 (3): 298–301. doi:10.1021/jm000406m. PMID 11462970.
- ↑ 26.0 26.1 26.2 Nagel J, Belozertseva I, Greco S, Kashkin V, Malyshkin A, Jirgensons A, Shekunova E, Eilbacher B, Bespalov A, Danysz W (December 2006). "Effects of NAAG peptidase inhibitor 2-PMPA in model chronic pain - relation to brain concentration". Neuropharmacology 51 (7–8): 1163–71. doi:10.1016/j.neuropharm.2006.07.018. PMID 16926034.
- ↑ Chen SR, Wozniak KM, Slusher BS, Pan HL (February 2002). "Effect of 2-(phosphono-methyl)-pentanedioic acid on allodynia and afferent ectopic discharges in a rat model of neuropathic pain". J. Pharmacol. Exp. Ther. 300 (2): 662–7. doi:10.1124/jpet.300.2.662. PMID 11805230.
- ↑ Tolias C, Wasserberg J (2002). "Critical decision making in severe head injury management". Trauma 4 (4): 211–221. doi:10.1191/1460408602ta246oa.
- ↑ "What is Stroke". National Stroke Association. Retrieved 2009-01-15.
- ↑ "Schizophrenia,". National Mental Health Information Center. Retrieved 2009-01-15.
- ↑ "Diabetic Neuropathies: The Nerve Damage of Diabetes". National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. 2009. Retrieved 2009-01-15.
- ↑ Berent-Spillson A, Robinson AM, Golovoy D, Slusher B, Rojas C, Russell JW (April 2004). "Protection against glucose-induced neuronal death by NAAG and GCP II inhibition is regulated by mGluR3". J. Neurochem. 89 (1): 90–9. doi:10.1111/j.1471-4159.2003.02321.x. PMID 15030392.
External links
- The MEROPS online database for peptidases and their inhibitors: M20.001
- Protein Data Bank: Protein Data Bank
- Glutamate carboxypeptidase II at the US National Library of Medicine Medical Subject Headings (MeSH)
| |||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||