Gitoformate
 | |
| Systematic (IUPAC) name | |
|---|---|
| [3-[5-(4,5-diformyloxy-6-methyloxan-2-yl)oxy-4-formyloxy-6-methyloxan-2-yl]oxy-6-[[16-formyloxy-14-hydroxy-10,13-dimethyl-17-(5-oxo-2H-furan-3-yl)-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-3-yl]oxy]-2-methyloxan-4-yl]formate | |
| Clinical data | |
| Trade names | Dynocard |
| Identifiers | |
|
10176-39-3 | |
| C01AA09 | |
| PubChem | CID 65598 |
| ChemSpider |
16736524 |
| UNII |
B69U29O7J9 |
| KEGG |
D07147 |
| Synonyms | Gitoxin 3',3'',3''',4''',16-pentaformate |
| Chemical data | |
| Formula | C46H64O19 |
| 920.989 g/mol | |
|
SMILES
| |
| |
| | |
Gitoformate (INN, or pentaformylgitoxin, trade name Dynocard) is a cardiac glycoside, a type of drug that can be used in the treatment of congestive heart failure and cardiac arrhythmia (irregular heartbeat).[1] Produced by Madaus, it is not available in the US, and does not seem to be available in Europe either.
Chemistry
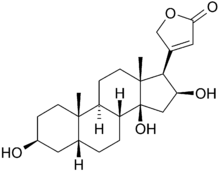
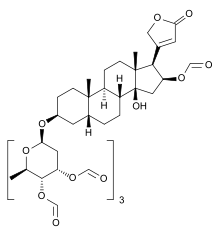
Gitoformate is a derivative of the glycoside gitoxin, with five of the six free hydroxyl groups formylated, one on the aglycon and four on the sugar.[2][3] Gitoxin, a cardiac glycoside from the Woolly Foxglove (Digitalis lanata), has an aglycon of the cardenolide type named gitoxigenin, which is also the aglycon of lanatoside B, another Digitalis lanata glycoside.[4]
References
- ↑ Rietbrock, N.; Woodcock, B. G.; Hrazdil, U. (1984). "Gitoformate and digitoxin as alternatives to kidney-dependent glycosides in the therapy of cardiac insufficiency". Arzneimittel-Forschung 34 (8): 915–917. PMID 6541927.
- ↑ Dei Cas, L.; Affatato, A.; Buia, E.; Casciarri, G.; Faggiano, P.; Giunti, G.; Metra, M.; Pelagatti, T.; Quinzanini, M. (1984). "Plasma levels of gitoxin (by RIA and rubidium-86 uptake) and systolic time after treatment with a single dose of gitoformate". Cardiologia (Rome, Italy) 29 (5–6): 291–300. PMID 6542412.
- ↑ PubChem 91540
- ↑ William O. Foye,Thomas L. Lemke, David A. Williams. Foye's principles of medicinal chemistry. p. 699. ISBN 978-0-7817-6879-5.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||