Gemeprost
 | |
| Systematic (IUPAC) name | |
|---|---|
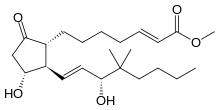
| methyl (2E,11α,13E,15R)-11,15-dihydroxy-16,16-dimethyl-9-oxoprosta-2,13-dien-1-oate | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Pessary | |
| Identifiers | |
|
64318-79-2 | |
| G02AD03 | |
| PubChem | CID 5282237 |
| DrugBank |
DB08964 |
| ChemSpider |
4445416 |
| UNII |
45KZB1FOLS |
| KEGG |
D02073 |
| Synonyms | methyl (E)-7-[(1R,2S,3R)-3-hydroxy-2-[(E,3R)-3-hydroxy-4,4-dimethyl-oct-1-enyl]-5-oxo-cyclopentyl]hept-2-enoate |
| Chemical data | |
| Formula | C23H38O5 |
| 394.545 g/mol | |
|
SMILES
| |
| |
| | |
Gemeprost (16, 16-dimethyl-trans-delta2 PGE1 methyl ester) is an analogue of prostaglandin E1.
Clinical use
It is used as a treatment for obstetric bleeding.
It is used with mifepristone to terminate pregnancy up to 24 weeks gestation. [1]
Side effects
Vaginal bleeding, cramps, nausea, vomiting, loose stools or diarrhea, headache, muscle weakness; dizziness; flushing; chills; backache; dyspnoea; chest pain; palpitations and mild pyrexia. Rare: Uterine rupture, severe hypotension, coronary spasms with subsequent myocardial infarctions.
References
- ↑ Bartley J, Brown A, Elton R, Baird DT (October 2001). "Double-blind randomized trial of mifepristone in combination with vaginal gemeprost or misoprostol for induction of abortion up to 63 days gestation". Human reproduction (Oxford, England) 16 (10): 2098–102. doi:10.1093/humrep/16.10.2098. PMID 11574498. Retrieved 2008-10-29.
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||