Gas gangrene
| Gas gangrene | |
|---|---|
 Photograph before right leg amputation (hemipelvectomy) of a patient with gas gangrene. The right thigh is edematous (swollen) and discoloured with necrotic bullae (large blisters). Crepitation is detected on deep palpation. At this juncture, the patient is in shock. | |
| Classification and external resources | |
| ICD-10 | A48.0 |
| ICD-9 | 040.0 |
| DiseasesDB | 31141 |
| MedlinePlus | 000620 |
| eMedicine | article/217943 article/782709 article/214992 |
| MeSH | D005738 |
Gas gangrene (also known as clostridial myonecrosis[1] and myonecrosis[2]) is a bacterial infection that produces gas in tissues in gangrene. This deadly form of gangrene usually is caused by Clostridium perfringens bacteria. It is a medical emergency.
Myonecrosis is a condition of necrotic damage, specific to muscle tissue. It is often seen in infections with C. perfringens or any of myriad soil-borne anaerobic bacteria. Bacteria cause myonecrosis by specific exotoxins. These microorganisms are opportunistic and, in general, enter the body through significant skin breakage. Gangrenous infection by soil-borne bacteria was common in the combat injuries of soldiers well into the 20th century, because of nonsterile field surgery and the basic nature of care for severe projectile wounds.[3]
Other causes of myonecrosis include envenomation by snakes of the Bothrops genus (family Viperidae), ischemic necrosis, caused by vascular blockage (e.g., diabetes type II), tumours that block or hoard blood supply, and disseminated intravascular coagulation or other thromboses.
Features
Gas gangrene can cause myonecrosis (muscle tissue death), gas production, and sepsis. Progression to toxemia and shock is often very rapid. It can easily be noticed by the large, blackened sores that form, as well as a degree of loud and distinctive crepitus caused by gas escaping the necrotic tissue.
Pathophysiology
Gas gangrene is caused by exotoxin-producing Clostridium species (most often C. perfringens, and C. novyi,[4] but less commonly C. septicum[5] or C. ramnosum),[6] which are mostly found in soil, but also found as normal gut flora, and other anaerobes (e.g., Bacteroides and anaerobic streptococci). The exotoxin is commonly found in C. perfringens type A strain and is known as alpha toxin. These environmental bacteria may enter the muscle through a wound and go on to proliferate in necrotic tissue and secrete powerful toxins. These toxins destroy nearby tissue, generating gas at the same time.
Other organisms may occasionally cause gas gangrene (for example, Klebsiella pneumoniae in the context of diabetes).[7]
A gas composition of 5.9% hydrogen, 3.4% carbon dioxide, 74.5% nitrogen and 16.1% oxygen was reported in one clinical case.[8]
Myonecrosis differs slightly from other types of necrosis. While the underlying causes are almost identical, the type of affected tissue (in particular, muscle tissue) is significantly more important for the patient's general health. Superficial necrosis is unsightly and can lead to unattractive scarring, but otherwise does not affect the patient's likelihood of survival or physical capability to the same extent. However, massive myonecrosis will likely result in the loss of movement of the entire region. If the necrotic damage is allowed to continue throughout an affected limb, then often that entire limb is lost permanently.
It is often difficult to identify the extent of muscle damage, as C. perfringens may be at work in deeper fascial layers below the skin. Unlike other anaerobic infections, discharge in these infections is often not purulent (filled with pus). Instead, the discharge is often described as "sweetly putrid" or "dishwater pus" because it is much thinner than normal pus. This is due to the lysis of neutrophils, a type of white blood cell, caused by the lecithinases and other toxins released by Clostridium species.
Soil-borne anaerobes are particularly well-adapted to surviving harsh conditions. Often, a scarcity of nutrition and competition for resources from numerous other species occurs. Changes in pH and temperature are often significant, also. Bacteria often possess the ability to create exotoxins to assist them in competing with other microbes in their natural environments. When such bacteria are able to enter a living host, they encounter a vast supply of nutrients, warm conditions, and an abundance of water. This enables the microbes to rapidly proliferate, far in excess of the immune system's capability to defend, as prokaryotic bacteria possess a far greater capacity for multiplication than the host's immune system. The combination of bacterial load and ability to multiply is the basis for the microbes' ability to cause massive infection. Alongside such rapid proliferation is a corresponding mass-production of exotoxin that causes severe damage to local tissue in the host. One such exotoxin is produced by C. perfringens and is responsible for the disease manifestations. This exotoxin is known as alpha toxin.[9]
Massive infection, gross injury, and depletion of the host's immune capability result in system-wide sepsis. This is partly due to the burden on the immune system, its corresponding release of inflammatory cytokines, and the distribution of bacterial toxins. Massive infection is likely to result in death from a combination of system-wide septic shock and the unintentionally damaging effects of the immune response. In animals, disability and distress caused by all of these factors markedly increase the chance of predation.
Treatment
Treatment is usually debridement and excision, with amputation necessary in many cases. Antibiotics alone are not effective because they do not penetrate ischaemic muscles sufficiently to be effective. However, penicillin is given as an adjuvant treatment to surgery. In addition to surgery and antibiotics, hyperbaric oxygen therapy is used and acts to inhibit the growth of and kill the anaerobic C. perfringens.[10][11]
Additional images

(a) Macroscopic picture of the edematous intestinal wall with multiple submucosal and subserosal cysts (b) Histological picture of the intestinal mucosa with nonreactive necrosis (c) Gram stain of cysts with large, rod-shaped bacteria (d) Electron microscopic picture of a bacterium found in a submucosal cyst
-
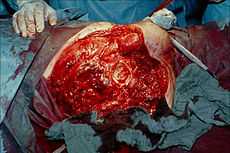
Hemipelvectomy for gas gangrene
-

Muscle biopsy examined under the microscope (haematoxylin-eosin stain, zoom 100×): the large white areas between the muscle fibers are due to gas formation.
-

Gram stain of a muscle biopsy showing Gram-positive, rod-shaped, anaerobic, spore-forming bacteria in the infected muscle tissue: The result is highly compatible with an infection with C. perfringens.
-

Gas gangrene of the shoulder.
See also
- Blackleg (disease) (a similar disease in livestock)
- List of cutaneous conditions
References
- ↑ James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. p. 269. ISBN 0-7216-2921-0.
- ↑ Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. ISBN 1-4160-2999-0.
- ↑ Pailler JL, Labeeu F (1986). "[Gas gangrene: a military disease?]". Acta Chir. Belg. (in French) 86 (2): 63–71. PMID 3716723.
- ↑ Hatheway CL (January 1990). "Toxigenic clostridia" (PDF). Clin. Microbiol. Rev. 3 (1): 66–98. PMC 358141. PMID 2404569.
- ↑ Bratton SL, Krane EJ, Park JR, Burchette S (1992). "Clostridium septicum infections in children". Pediatr Infect Dis J 11 (7): 569–75. doi:10.1097/00006454-199207000-00011. PMID 1528648.
- ↑ van der Vorm ER, von Rosenstiel IA, Spanjaard L, Dankert J (April 1999). "Gas gangrene in an immunocompromised girl due to a Clostridium ramosum infection". Clin. Infect. Dis. 28 (4): 923–4. doi:10.1086/517249. PMID 10825071.
- ↑ Chang C-W, Wang MD T-E, Shih S-C, Chang W-H, Chen M-J (2008). "Shortness of breath, fever—and pain in both legs". Lancet 372 (9648): 1518. doi:10.1016/S0140-6736(08)61621-9. PMID 18970978.
- ↑ ^ Chi CH, Chen KW, Huang JJ, Chuang YC, Wu MH (1995). "Gas composition in Clostridium septicum gas gangrene". J Formos Med Assoc 94 (12): 757–9. PMID 8541740.
- ↑ Awad MM, Bryant AE, Stevens DL, Rood JI (January 1995). "Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene". Mol. Microbiol. 15 (2): 191–202. doi:10.1111/j.1365-2958.1995.tb02234.x. PMID 7746141.
- ↑ Hart GB, Strauss MB (1990). "Gas Gangrene — Clostridial Myonecrosis: A Review". J. Hyperbaric Med 5 (2): 125–144. Retrieved 2008-05-16.
- ↑ Zamboni WA, Riseman JA, Kucan JO (1990). "Management of Fournier's Gangrene and the role of Hyperbaric Oxygen". J. Hyperbaric Med 5 (3): 177–186. Retrieved 2008-05-16.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||