Gadobutrol
 | |
| Systematic (IUPAC) name | |
|---|---|
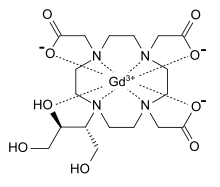
| gadolinium(III) 2,2',2''-(10-((2R,3S)-1,3,4-trihydroxybutan-2-yl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetate | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Licence data | US FDA:link |
| |
| |
| IV | |
| Identifiers | |
|
138071-82-6 | |
| V08CA09 | |
| PubChem | CID 72057 |
| DrugBank |
DB06703 |
| UNII |
1BJ477IO2L |
| KEGG |
D07420 |
| ChEBI |
CHEBI:68841 |
| ChEMBL |
CHEMBL1628503 |
| Chemical data | |
| Formula | C18H31GdN4O9 |
| 604.710 g/mol | |
|
SMILES
| |
| | |
Gadobutrol (INN) (Gd-DO3A-butrol) is a gadolinium-based MRI contrast agent (GBCA).
It received marketing approval in Canada[1] and in the United States.[2][3][4]
As of 2007, it was the only GBCA approved at 1.0 molar concentrations.[5]
Gadobutrol is marketed by Bayer Schering Pharma as Gadovist, and by Bayer HealthCare Pharmaceuticals as Gadavist.[6]
References
- ↑ Cheng, KT (2007). Gadobutrol. Molecular Imaging and Contrast Agent Database (MICAD) (Bethesda, MD: National Center for Biotechnology Information (NCBI)). PMID 20641787. NBK23589.
- ↑ http://bayerimaging.com/products/gadavist/index.php
- ↑ "FDA approves imaging agent for central nervous system scans" (Press release). U.S. Food and Drug Administration (FDA). March 15, 2011. Retrieved March 31, 2011.
- ↑ "U.S. FDA Approves Bayer’s Gadavist (Gadobutrol) Injection for MRI of the Central Nervous System" (Press release). Bayer HealthCare Pharmaceuticals. March 14, 2011. Retrieved March 31, 2011.
- ↑ "Gadobutrol 1.0-molar in Cardiac Magnetic Resonance Imaging (MRI) - Further Enhancing the Capabilities of Contrast-enhanced MRI in Ischaemic and Non-ischaemic Heart Disease?"
- ↑ "Gadavist full prescribing information". Retrieved 2011-03-14.
| ||||||||||||||||||||||||||||||||||||||||||||||||