Fulvalene
A fulvalene is a hydrocarbon obtained by formally cross-conjugating two rings through a common exocyclic double bond.[1] The name is derived from the similarly structured fulvenes which lack one ring. Triapentafulvalene (3) is also known as calicene as in calix or chalice because of its wine-glass appearance.
In general, the parent fulvalenes are very unstable; for instance, the parent triafulvalene (1) has never been synthesized. On the other hand stable fulvalenes can be obtained by proper substitution or benzannulation. Several members should be stabilized taking into account a dipolar mesomeric form with for instance pentaheptafulvalene 4, which can be thought of as a tropylium cation joined to a cyclopentadienyl anion (both stable and aromatic). In this compound the dipolar structure is calculated to contribute 23% to the total structure.
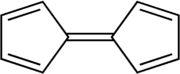
Pentafulvalene
 | |
 | |
| Names | |
|---|---|
| Other names
Bicyclopentyliden-2,4,2',4'-tetraene 1,1'-Bi[cylopentadienylidene] Pentafulvalene Bicyclopentadienylidene [5,5']Bicyclopentadienylidene | |
| Identifiers | |
| 91-12-3 | |
| ChEBI | CHEBI:51994 |
| ChemSpider | 9083553 |
| |
| Jmol-3D images | Image |
| PubChem | 10908294 |
| |
| Properties | |
| Molecular formula |
C10H8 |
| Molar mass | 128.17 g·mol−1 |
| Density | 1.129 g/ml |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Pentafulvalene is the member of the fulvalene family with the molecular formula C10H8. It is of theoretical interest as one of the simplest non-benzenoid conjugated hydrocarbons. Fulvalene is an unstable isomer of the more common benzenoid aromatic compounds naphthalene and azulene. It is also known as bicyclopentadienylidene. Pentafulvalene consists of two 5-membered rings, each with two double bonds, joined by yet a fifth double bond. It has D2h symmetry.
An earlier attempt at synthesis of pentafulvalene in 1951 by Pauson and Kealy resulted in the accidental discovery of ferrocene.[2] Its synthesis was first reported in 1958 by E. A. Matzner at Yale University, working under William von Eggers Doering.[3] In this method, dissertation only and never published, a cyclopentadienyl anion is coupled with iodine to the dihydrofulvalene which is then doubly deprotonated with n-butyllithium to the dianion and then oxidized with oxygen. Pentafulvalene was spectroscopically observed at 77 K from photolysis of diazocyclopentadiene (dimerization of two cyclopentadiene carbenes) [4] with UV spectra matching those obtained by the Doering group. Final isolation of the compound came in 1986 [5] via a method similar to that of Doering. The compound was found to be nonaromatic and extremely reactive above −50 °C through Diels-Alder dimerization.
Perchlorofulvalene C10Cl8 is quite stable in contrast to the hydrocarbon.[6] Tetrathiafulvalene is an organic semiconductor.
Fulvalenes as a ligand
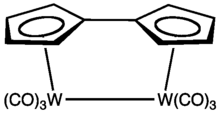
Fulvalenes forms stable organometallic complexes that can be formally considered derivatives of the dianion C10H82−. In fact as mentioned above, ferrocene was isolated from an attempted synthesis of fulvalene. Many compounds are known, especially for the early transition metals.[7] The central C-C bond in some fulvalene complexes can break reversibly.[8]
References
- ↑ The Fulvalenes Brian Halton Eur. J. Org. Chem. 2005, 3391–3414 doi:10.1002/ejoc.200500231
- ↑ T. J. Kealy, P. L. Pauson (1951). "A New Type of Organo-Iron Compound". Nature 168 (4285): 1039. doi:10.1038/1681039b0.
- ↑ Dissertation Abstracts Int'l 26-06 page 3270 6411876.
- ↑ Photochemical Experiments in Rigid Media at Low Temperatures. II. The Reactions of Methylene, Cyclopentadienylene and Diphenylmethylene William B. DeMore, H. O. Pritchard, Norman Davidson J. Am. Chem. Soc., 1959, 81 (22), pp 5874–5879 doi:10.1021/ja01531a008
- ↑ Synthese von Pentafulvalen durch oxidative Kupplung von Cyclopentadienid mittels Kupfer(II)-chlorid André Escher, Werner Rutsch, Markus Neuenschwander Helvetica Chimica Acta Volume 69 Issue 7, Pages 1644 - 1654 1986 doi:10.1002/hlca.19860690719
- ↑ Mark, V. “Perchlorofulvalene” Organic Syntheses, Collected Volume 5, p.901 (1973). http://www.orgsyn.org/orgsyn/pdfs/CV5P0901.pdf
- ↑ Marta González-Maupoey, Vanessa Tabernero and Tomás Cuenca "Early transition metal fulvalene complexes" Coordination Chemistry Reviews 2009, Volume 253, Pages 1854-1881. doi:10.1016/j.ccr.2009.02.013
- ↑ Boese, R. J.; Cammack, K.; Matzger, A. J.; Pflug, K.; Tolman,W. B.; Vollhardt, K. P. C.; Weidman, T. W. "Photochemistry of (Fulvalene)tetracarbonyldiruthenium and Its Derivatives: Efficient Light Energy Storage Devices" Journal of the American Chemistry Society 1997, volume 119, p. 6757-6773. doi:10.1021/ja9707062