Fracture toughness
In materials science, fracture toughness is a property which describes the ability of a material containing a crack to resist fracture, and is one of the most important properties of any material for many design applications. The linear-elastic fracture toughness of a material is determined from the stress intensity factor (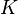 ) at which a thin crack in the material begins to grow. It is denoted KIc and has the units of
) at which a thin crack in the material begins to grow. It is denoted KIc and has the units of 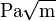 or
or 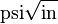 . Plastic-elastic fracture toughness is denoted by JIc, with the unit of J/cm2 or lbf-in/in2, and is a measurement of the energy required to grow a thin crack.
. Plastic-elastic fracture toughness is denoted by JIc, with the unit of J/cm2 or lbf-in/in2, and is a measurement of the energy required to grow a thin crack.
The subscript Ic denotes mode I crack opening under a normal tensile stress perpendicular to the crack, since the material can be made deep enough to stand shear (mode II) or tear (mode III).
Fracture toughness is a quantitative way of expressing a material's resistance to brittle fracture when a crack is present. If a material has much fracture toughness it will probably undergo ductile fracture. Brittle fracture is very characteristic of materials with less fracture toughness.[1]
Fracture mechanics, which leads to the concept of fracture toughness, was broadly based on the work of A. A. Griffith who, among other things, studied the behavior of cracks in brittle materials.
A related concept is the work of fracture (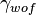 ) which is directly proportional to
) which is directly proportional to 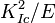 , where
, where 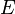 is the Young's modulus of the material.[2] Note that, in SI units,
is the Young's modulus of the material.[2] Note that, in SI units, 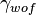 is given in J/m2.
is given in J/m2.
Example values
The following table shows some typical values of fracture toughness for various materials:
| Material type | Material | KIc (MPa · m1/2) |
|---|---|---|
| Metal | Aluminum alloy (7075) | 24 |
| Steel alloy (4340) | 50 | |
| Titanium alloy | 44–66 | |
| Aluminum | 14–28 | |
| Ceramic | Aluminum oxide | 3–5 |
| Silicon carbide | 3–5 | |
| Soda-lime glass | 0.7–0.8 | |
| Concrete | 0.2–1.4 | |
| Polymer | Polymethyl methacrylate | 0.7–1.6 |
| Polystyrene | 0.7–1.1 | |
| Composite | Mullite-fibre composite | 1.8–3.3[3] |
| Silica aerogels | 0.0008–0.0048[4] |
Crack growth as a stability problem
Consider a body with flaws (cracks) that is subject to some loading; the stability of the crack can be assessed as follows. We can assume for simplicity that the loading is of constant displacement or displacement controlled type (such as loading with a screw jack); we can also simplify the discussion by characterizing the crack by its area, A. If we consider an adjacent state of the body as being one with a broader crack (area A+dA), we can then assess strain energy in the two states and evaluate strain energy release rate.
The rate is reckoned with respect to the change in crack area, so if we use U for strain energy, the strain energy release rate is numerically dU/dA. It may be noted that for a body loaded in constant displacement mode, the displacement is applied and the force level is dictated by stiffness (or compliance) of the body. If the crack grows in size, the stiffness decreases, so the force level will decrease. This decrease in force level under the same displacement (strain) level indicates that the elastic strain energy stored in the body is decreasing—is being released. Hence the term strain energy release rate which is usually denoted with symbol G.
The strain energy release rate is higher for higher loads and broader cracks. If the strain energy so released exceeds a critical value Gc, then the crack will grow spontaneously. For brittle materials, Gc can be equated to the surface energy of the (two) new crack surfaces; in other words, in brittle materials, a crack will grow spontaneously if the strain energy released is equal to or more than the energy required to grow the crack surface(s). The stability condition can be written as
- elastic energy released = surface energy created.
If the elastic energy released is less than the critical value, then the crack will not grow; equality signifies neutral stability and if the strain energy release rate exceeds the critical value, the crack will start growing in an unstable manner. For ductile materials, energy associated with plastic deformation has to be taken into account. When there is plastic deformation at the crack tip (as occurs most often in metals) the energy to propagate the crack may increase by several orders of magnitude as the work related to plastic deformation may be much larger than the surface energy. In such cases, the stability criterion has to be restated as
- elastic energy released = surface energy + plastic deformation energy.
Practically, this means a higher value for the critical value Gc. From the definition of G, we can deduce that it has dimensions of work (or energy) /area or force/length. For ductile metals GIc is around 50–200 kJ/m2, for brittle metals it is usually 1–5 and for glasses and brittle polymers it is almost always less than 0.5.
The problem can also be formulated in terms of stress instead of energy, leading to the terms stress intensity factor K (or KI for mode I) and critical stress intensity factor Kc (and KIc). These Kc and KIc (etc.) quantities are commonly referred to as fracture toughness, though it is equivalent to use Gc. Typical values for KIcare 150 MN/m3/2 for ductile (very tough) metals, 25 for brittle ones and 1–10 for glasses and brittle polymers. Notice the different units used by GIc and KIc. Engineers tend to use the latter as an indication of toughness.
Conjoint action
There are number of instances where this picture of a critical crack is modified by corrosion. Thus, fretting corrosion occurs when a corrosive medium is present at the interface between two rubbing surfaces. Fretting (in the absence of corrosion) results from the disruption of very small areas that bond and break as the surfaces undergo friction, often under vibrating conditions. The bonding contact areas deform under the localised pressure and the two surfaces gradually wear away. Fracture mechanics dictates that each minute localised fracture has to satisfy the general rule that the elastic energy released as the bond fractures has to exceed the work done in plastically deforming it and in creating the (very tiny) fracture surfaces. This process is enhanced when corrosion is present, not least because the corrosion products act as an abrasive between the rubbing surfaces.
Fatigue is another instance where cyclical stressing, this time of a bulk lump of metal, causes small flaws to develop. Ultimately one such flaw exceeds the critical condition and fracture propagates across the whole structure. The fatigue life of a component is the time it takes for criticality to be reached, for a given regime of cyclical stress. Corrosion fatigue is what happens when a cyclically stressed structure is subjected to a corrosive environment at the same time. This not only serves to initiate surface cracks but (see below) actually modifies the crack growth process. As a result the fatigue life is shortened, often considerably.
Stress-corrosion cracking (SCC)
This phenomenon is the unexpected sudden failure of normally ductile metals subjected to a constant tensile stress in a corrosive environment. Certain austenitic stainless steels and aluminium alloys crack in the presence of chlorides, mild steel cracks in the presence of alkali (boiler cracking) and copper alloys crack in ammoniacal solutions (season cracking). Worse still, high-tensile structural steels crack in an unexpectedly brittle manner in a whole variety of aqueous environments, especially chloride. With the possible exception of the latter, which is a special example of hydrogen cracking, all the others display the phenomenon of subcritical crack growth; i.e. small surface flaws propagate (usually smoothly) under conditions where fracture mechanics predicts that failure should not occur. That is, in the presence of a corrodent, cracks develop and propagate well below KIc. In fact, the subcritical value of the stress intensity, designated as KIscc, may be less than 1% of KIc, as the following table shows:
| Alloy | KIc (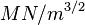 ) ) | SCC environment | KIscc (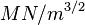 ) ) |
| 13Cr steel | 60 | 3% NaCl | 12 |
| 18Cr-8Ni | 200 | 42% MgCl2 | 10 |
| Cu-30Zn | 200 | NH4OH, pH7 | 1 |
| Al-3Mg-7Zn | 25 | aqueous halides | 5 |
| Ti-6Al-4V | 60 | 0.6M KCl | 20 |
The subcritical nature of propagation may be attributed to the chemical energy released as the crack propagates. That is,
- elastic energy released + chemical energy = surface energy + deformation energy.
The crack initiates at KIscc and thereafter propagates at a rate governed by the slowest process, which most of the time is the rate at which corrosive ions can diffuse to the crack tip. As the crack advances so K rises (because crack size appears in the calculation of stress intensity). Finally it reaches KIc, whereupon swift fracture ensues and the component fails. One of the practical difficulties with SCC is its unexpected nature. Stainless steels, for example, are employed because under most conditions they are passive; i.e. effectively inert. Very often one finds a single crack has propagated whiles the left metal surface stays apparently unaffected.
Toughening Mechanisms
Intrinsic Mechanisms
Intrinsic toughening mechanisms are processes which act ahead of the crack tip to increase the material's toughness. These will tend to be related to the structure and bonding of the base material, as well as microstructural features and additives to it. Examples of mechanisms include crack deflection by secondary phases, crack bifurcation due to fine grain structure and modification to the grain boundaries, and crack meandering by pores in the material. Any alteration to the base material which increases its ductility can also be thought of as intrinsic toughening.[5]
Extrinsic Mechanisms
Extrinsic toughening mechanisms are processes which act behind the crack tip to resist its further opening. Examples include fibre/lamella bridging, where these structures hold the two fracture surfaces together after the crack has propagated through the matrix, crack wedging from the friction between two rough fracture surfaces, microcracking, where smaller cracks form in the material around the main crack, relieving the stress at the crack tip by effectively increasing the material's compliance, and transformation toughening.[6]
"Transformation toughening" is a phenomenon whereby a material undergoes one or more martensitic (displacive, diffusionless) phase transformations which result in an almost instantaneous change in volume of that material. This transformation is triggered by a change in the stress state of the material, such as an increase in tensile stress, and acts in opposition to the applied stress. Thus when the material is locally put under tension, for example at the tip of a growing crack, it can undergo a phase transformation which increases its volume, lowering the local tensile stress and hindering the crack's progression through the material. This mechanism is exploited to increase the toughness of ceramic materials, most notably in Yttria-stabilized zirconia for applications such as ceramic knives and thermal barrier coatings on jet engine turbine blades.[7]
Fracture toughness testing methods
Fracture toughness is a critical mechanical property for certain applications. There are several types of test used to measure fracture toughness of materials.
Determination of plane strain fracture toughness, KIc
When a material behaves in a linear elastic way prior to failure, such that the plastic zone is small compared to the specimen dimension, a critical value of Mode-I stress intensity factor can be an appropriate fracture parameter. This method provides a quantitative measure of fracture toughness in terms of the critical plane strain stress intensity factor. The test must be validated once complete to ensure the results are meaningful. The specimen size is fixed, and must be large enough to ensure plane strain conditions at the crack tip. This limits the product forms to which the test can be applied. In the 1960s, it was postulated that small specimens or thin sections fail under plane stress conditions, and that ‘‘plane strain fracture’’ occurs in thick sections. The ASTM E 399 test method reflects this viewpoint. Over the years, it has been taken as an indisputable fact that toughness decreases with increasing specimen size until a plateau is reached. Specimen size requirements in ASTM E 399 are intended to ensure that KIc measurements correspond to the supposed plane strain plateau. The specimen size requirements in this standard are far more stringent than they need to be to ensure predominately plane strain conditions at the crack tip. The real key to a K-based test method is ensuring that the specimen fractures under nominally linear elastic conditions. That is, the plastic zone must be small compared to the specimen cross section. Consequently, the important specimen dimensions to ensure a valid K test are the crack length a and the ligament length W – a, not the thickness B. Four specimen configurations are permitted by the current version of E 399: the compact, SE(B), arc-shaped, and disk-shaped specimens. Specimens for KIc tests are usually fabricated with the width W equal to twice the thickness B. They are fatigue precracked so that the crack length/width ratio (a /W) lies between 0.45 and 0.55. Thus, the specimen design is such that all of the key dimensions, a, B, and W− a, are approximately equal. This design results in the efficient use of material, since the standard requires that each of these dimensions must be large compared to the plastic zone.
Determination of tear resistance (Kahn tear test)
The tear test (e.g. Kahn tear test) provides a semi-quantitative measure of toughness in terms of tear resistance. This type of test requires a smaller specimen, and can therefore be used for a wider range of product forms. The tear test can also be used for very ductile aluminium alloys (e.g. 1100, 3003), where linear elastic fracture mechanics do not apply (see properties in practice).
Fracture toughness of AISI steel
The fracture toughness of AISI 4340 steel has been determined by several methods, i.e. (i)Jr curve, (ii)δr curve, (iii) Kr curve, (iv) stretch zone size measurements (v) non-linear energy method of Poulose et al. and by (vi) a new procedure proposed recently by Banerjee. Compact tension specimens with TL orientation have been used. All the specimens used satisfied the ASTM E813 test size requirements. Applicability of various fracture toughness estimation procedures like (i) Hanhn and Rosenfield, (ii) Rolfe and Barsom and (iii) equivalent energy rate method of Bucci et al. have been examined. These values have been compared with true fracture toughness of the material obtained by ASTM E399 test procedure.[8]
Comparison of various conventional test methods indicate multiple specimen curve method gives most consistent results and these values are within +15% of the true fracture toughness value. Out of all estimation procedures Rolfe and Barsom's method appears to be best, giving number within +8% of the true fracture toughness value. Non-linear energy method was found to give a fracture toughness value consistent with true fracture toughness of the material
Other methods for determining fracture toughness
- C1161 Test Method for Flexural Strength of Advanced Ceramics at Ambient Temperature
- C1322 Practice for Fractography and Characterization of Fracture Origins in Advanced Ceramics
- E4 Practices for Force Verification of Testing Machines
- E112 Test Methods for Determining Average Grain Size
- E177 Practice for Use of the Terms Precision and Bias in ASTM Test Methods
- E337 Test Method for Measuring Humidity with a Psychrometer (the Measurement of Wet- and Dry-Bulb Temperatures)
- E399 Test Method for Plain-strain Fracture Toughness of Metallic Materials
- E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
- E740 Practice for Fracture Testing with Surface-Crack Tension Specimens
- E1823 Terminology Relating to Fatigue and Fracture Testing
- IEEE/ASTM SI 10 Standard for Use of the International System of Units (SI) (The Modern Metric System)[9]
See also
- Stress intensity factor
- Puncture resistance
- Fracture mechanics
- Brittle-ductile transition zone
- Charpy impact test
- Impact (mechanics)
- Izod impact strength test
- Toughness of ceramics by indentation
- Shock (mechanics)
- Stress corrosion cracking
References
- ↑ Hertzberg, Richard W. (December 1995). Deformation and Fracture Mechanics of Engineering Materials (4 ed.). Wiley. ISBN 0-471-01214-9.
- ↑ Sérgio Francisco dos Santos, José de Anchieta Rodrigues (2003). "Correlation Between Fracture Toughness, Work of Fracture and Fractal Dimensions of Alumina-Mullite-Zirconia Composites". Materials Research 6 (2): 219–226. doi:10.1590/s1516-14392003000200017.
- ↑ AR Boccaccini, S Atiq, DN Boccaccini, I Dlouhy, C Kaya (2005). "Fracture behaviour of mullite fibre reinforced-mullite matrix composites under quasi-static and ballistic impact loading". Composites Science and Technology 65: 325–333. doi:10.1016/j.compscitech.2004.08.002.
- ↑ J. Phalippou, T. Woignier, R. Rogier (1989). "Fracture toughness of silica aerogels". Journal de Physique Colloques 50: C4–191. doi:10.1051/jphyscol:1989431.
- ↑ Wei, Robert (2010), Fracture Mechanics: Integration of Mechanics, Materials Science and Chemistry, Cambridge University Press, retrieved 24 September 2014
- ↑ Liang, Yiling (2010), The toughening mechanism in hybrid epoxy-silica-rubber nanocomposites, Lehigh University, p. 20, retrieved 24 September 2014
- ↑ Padture, Nitin (12 April 2002). "Thermal Barrier Coatings for Gas-Turbine Engine Applications". Science 296: 280–284. doi:10.1126/science.1068609.
- ↑ Engineering Fracture Mechanics, Volume 25, Issue 4, 1986
- ↑ NIST SRM 2100 Fracture Toughness of Ceramics
Other references
- Anderson, T. L., Fracture Mechanics: Fundamentals and Applications (CRC Press, Boston 1995).
- Davidge, R. W., Mechanical Behavior of Ceramics (Cambridge University Press 1979).
- Lawn, B., Fracture of Brittle Solids (Cambridge University Press 1993, 2nd edition).
- Knott, Fundamentals of Fracture Mechanics (1973).
- Foroulis (ed.), Environmentally-Sensitive Fracture of Engineering Materials (1979).
- Suresh, S., Fatigue of Materials (Cambridge University Press 1998, 2nd edition).
- West, J. M., Basic Corrosion & Oxidation (Horwood 1986, 2nd edn), chap.12.
- Green, D. J.; Hannink, R.; Swain, M. V. (1989). Transformation Toughening of Ceramics, Boca Raton: CRC Press. ISBN 0-8493-6594-5.
- http://www.sv.vt.edu/classes/MSE2094_NoteBook/97ClassProj/exper/gordon/www/fractough.html
- http://www.springerlink.com/content/v2m7u4qm53172069/fulltext.pdf sriram