Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR)[1] is a technique which is used to obtain an infrared spectrum of absorption, emission, photoconductivity or Raman scattering of a solid, liquid or gas. An FTIR spectrometer simultaneously collects high spectral resolution data over a wide spectral range. This confers a significant advantage over a dispersive spectrometer which measures intensity over a narrow range of wavelengths at a time.
The term Fourier transform infrared spectroscopy originates from the fact that a Fourier transform (a mathematical process) is required to convert the raw data into the actual spectrum. For other uses of this kind of technique, see Fourier transform spectroscopy.
Conceptual introduction

The goal of any absorption spectroscopy (FTIR, ultraviolet-visible ("UV-Vis") spectroscopy, etc.) is to measure how well a sample absorbs light at each wavelength. The most straightforward way to do this, the "dispersive spectroscopy" technique, is to shine a monochromatic light beam at a sample, measure how much of the light is absorbed, and repeat for each different wavelength. (This is how UV-Vis spectrometers work, for example.)
Fourier transform spectroscopy is a less intuitive way to obtain the same information. Rather than shining a monochromatic beam of light at the sample, this technique shines a beam containing many frequencies of light at once, and measures how much of that beam is absorbed by the sample. Next, the beam is modified to contain a different combination of frequencies, giving a second data point. This process is repeated many times. Afterwards, a computer takes all these data and works backwards to infer what the absorption is at each wavelength.
The beam described above is generated by starting with a broadband light source—one containing the full spectrum of wavelengths to be measured. The light shines into a Michelson interferometer—a certain configuration of mirrors, one of which is moved by a motor. As this mirror moves, each wavelength of light in the beam is periodically blocked, transmitted, blocked, transmitted, by the interferometer, due to wave interference. Different wavelengths are modulated at different rates, so that at each moment, the beam coming out of the interferometer has a different spectrum.
As mentioned, computer processing is required to turn the raw data (light absorption for each mirror position) into the desired result (light absorption for each wavelength). The processing required turns out to be a common algorithm called the Fourier transform (hence the name, "Fourier transform spectroscopy"). The raw data is sometimes called an "interferogram".
Developmental background
The first low-cost spectrophotometer capable of recording an infrared spectrum was the Perkin-Elmer Infracord produced in 1957.[2] This instrument covered the wavelength range from 2.5 μm to 15 μm (wavenumber range 4000 cm−1 to 660 cm−1). The lower wavelength limit was chosen to encompass the highest known vibration frequency due to a fundamental molecular vibration. The upper limit was imposed by the fact that the dispersing element was a prism made from a single crystal of rock-salt (sodium chloride) which becomes opaque at wavelengths longer than about 15 μm; this spectral region became known as the rock-salt region. Later instruments used potassium bromide prisms to extend the range to 25 μm (400 cm−1) and caesium iodide 50 μm (200 cm−1). The region beyond 50 μm (200 cm−1) became known as the far-infrared region; at very long wavelengths it merges into the microwave region. Measurements in the far infrared needed the development of accurately ruled diffraction gratings to replace the prisms as dispersing elements since salt crystals are opaque in this region. More sensitive detectors than the bolometer were required because of the low energy of the radiation. One such was the Golay detector. An additional issue is the need to exclude atmospheric water vapour because water vapour has an intense pure rotational spectrum in this region. Far-infrared spectrophotometers were cumbersome, slow and expensive. The advantages of the Michelson interferometer were well-known, but considerable technical difficulties had to be overcome before a commercial instrument could be built. Also an electronic computer was needed to perform the required Fourier transform and this only became practicable with the advent of mini-computers, such as the PDP-8 which became available in 1965. Digilab pioneered the world's first commercial FTIR spectrometer (Model FTS-14) in 1969 [3] (Digilab FTIRs are now a part of Agilent technologies's molecular product line after it acquired spectroscopy business from Varian).[4][5]
Michelson interferometer
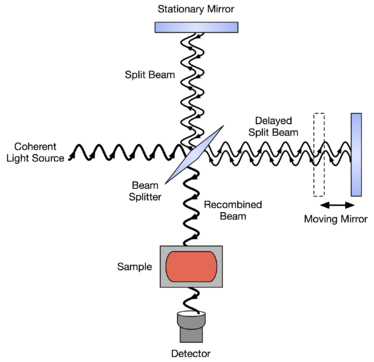
In a Michelson interferometer adapted for FTIR, light from the polychromatic infrared source, approximately a black-body radiator, is collimated and directed to a beam splitter. Ideally 50% of the light is refracted towards the fixed mirror and 50% is transmitted towards the moving mirror. Light is reflected from the two mirrors back to the beam splitter and (ideally) 50% of the original light passes into the sample compartment. There, the light is focused on the sample. On leaving the sample compartment the light is refocused on to the detector. The difference in optical path length between the two arms to the interferometer is known as the retardation. An interferogram is obtained by varying the retardation and recording the signal from the detector for various values of the retardation. The form of the interferogram when no sample is present depends on factors such as the variation of source intensity and splitter efficiency with wavelength. This results in a maximum at zero retardation, when there is constructive interference at all wavelengths, followed by series of "wiggles". The position of zero retardation is determined accurately by finding the point of maximum intensity in the interferogram. When a sample is present the background interferogram is modulated by the presence of absorption bands in the sample.
Advantages of Fourier Transform spectroscopy
There are two principal advantages for an FT spectrometer compared to a scanning (dispersive) spectrometer.[6][7]
- The multiplex or Fellgett's advantage. This arises from the fact that information from all wavelengths is collected simultaneously. It results in a higher Signal-to-noise ratio for a given scan-time or a shorter scan-time for a given resolution.
- The throughput or Jacquinot's advantage. This results from the fact that, in a dispersive instrument, the monochromator has entrance and exit slits which restrict the amount of light that passes through it. The interferometer throughput is determined only by the diameter of the collimated beam coming from the source.
Other minor advantages include less sensitivity to stray light because it would have little effect on the interferogram, while it would directly impinge on the detector in a dispersive instrument.[7] Another one is the "Connes' advantage" (better wavelength accuracy), because every scan can be calibrated with a helium-neon laser which has a stable and accurately known wavelength.[7] However, a disadvantage is that FTIR cannot use the advanced electronic filtering techniques that often makes its signal-to-noise ratio inferior to that of dispersive measurements.[7]
Theory
FTIR is a method of measuring an infrared absorption spectrum. For a discussion of why people measure infrared absorption spectra, i.e. why and how substances absorb infrared light, see the article: Infrared spectroscopy.
Resolution
The interferogram belongs in the length domain. Fourier transform (FT) inverts the dimension, so the FT of the interferogram belongs in the reciprocal length domain, that is the wavenumber domain. The spectral resolution in wavenumbers per cm is equal to the reciprocal of the maximum retardation in cm. Thus a 4 cm−1 resolution will be obtained if the maximum retardation is 0.25 cm; this is typical of the cheaper FTIR instruments. Much higher resolution can be obtained by increasing the maximum retardation. This is not easy as the moving mirror must travel in a near-perfect straight line. The use of corner-cube mirrors in place of the flat mirrors is helpful as an outgoing ray from a corner-cube mirror is parallel to the incoming ray, regardless of the orientation of the mirror about axes perpendicular to the axis of the light beam. Connes measured in 1966 the temperature of the atmosphere of Venus by recording the vibration-rotation spectrum of Venusian CO2 at 0.1 cm−1 resolution.[8] Michelson himself attempted to resolve the hydrogen Hα emission band in the spectrum of a hydrogen atom into its two components by using his interferometer.[1] p25 A spectrometer with 0.001 cm−1 resolution is now available commercially. The throughput advantage is important for high-resolution FTIR as the monochromator in a dispersive instrument with the same resolution would have very narrow entrance and exit slits.
Beam splitter
The beam-splitter can not be made of common types of glass, as they are opaque to infrared radiation of wavelengths longer than about 2.5 μm. A thin film, usually of a plastic material, is used instead. However, as any material has a limited range of optical transmittance, several beam-splitters are used interchangeably to cover a wide spectral range.It is either a partially silvered mirror or a thin film of germanium sandwiched between two KBr plates.
Fourier transform
The interferogram in practice consists of a set of intensities measured for discrete values of retardation. The difference between successive retardation values is constant. Thus, a discrete Fourier transform is needed. The fast Fourier transform (FFT) algorithm is used.
Far-infrared FTIR
The first FTIR spectrometers were developed for far-infrared range. The reason for this has to do with the mechanical tolerance needed for good optical performance, which is related to the wavelength of the light being used. For the relatively long wavelengths of the far infrared, ~10 μm tolerances are adequate, whereas for the rock-salt region tolerances have to be better than 1 μm. A typical instrument was the cube interferometer developed at the NPL[9] and marketed by Grubb Parsons. It used a stepper motor to drive the moving mirror, recording the detector response after each step was completed.
Mid-infrared FTIR
With the advent of cheap microcomputers it became possible to have a computer dedicated to controlling the spectrometer, collecting the data, doing the Fourier transform and presenting the spectrum. This provided the impetus for the development of FTIR spectrometers for the rock-salt region. The problems of manufacturing ultra-high precision optical and mechanical components had to be solved. A wide range of instruments are now available commercially. Although instrument design has become more sophisticated, the basic principles remain the same. Nowadays, the moving mirror of the interferometer moves at a constant velocity, and sampling of the interferogram is triggered by finding zero-crossings in the fringes of a secondary interferometer lit by a helium–neon laser. In modern FTIR systems the constant mirror velocity is not strictly required, as long as the laser fringes and the original interferogram are recorded simultaneously with higher sampling rate and then re-interpolated on a constant grid, as pioneered by James W. Brault. This confers very high wavenumber accuracy on the resulting infrared spectrum and avoids wavenumber calibration errors.
Near-infrared FTIR
The near-infrared region spans the wavelength range between the rock-salt region and the start of the visible region at about 750 nm. Overtones of fundamental vibrations can be observed in this region. It is used mainly in industrial applications such as process control and chemical imaging.
Applications
FTIR can be used in all applications where a dispersive spectrometer was used in the past (see external links). In addition, the multiplex and throughput advantages have opened up new areas of application. These include:
- GC-IR (gas chromatography-infrared spectrometry). A gas chromatograph can be used to separate the components of a mixture. The fractions containing single components are directed into an FTIR spectrometer, to provide the infrared spectrum of the sample. This technique is complementary to GC-MS (gas chromatography-mass spectrometry). The GC-IR method is particularly useful for identifying isomers, which by their nature have identical masses. The key to the successful use of GC-IR is that the interferogram can be captured in a very short time, typically less than 1 second. FTIR has also been applied to the analysis of liquid chromatography fractions.[7]
- TG-IR (thermogravimetry-infrared spectrometry) IR spectra of the gases evolved during thermal decomposition are obtained as a function of temperature.[10]
- Micro-samples. Tiny samples, such as in forensic analysis, can be examined with the aid of an infrared microscope in the sample chamber. An image of the surface can be obtained by scanning.[11] Another example is the use of FTIR to characterize artistic materials in old-master paintings.[12]
- Emission spectra. Instead of recording the spectrum of light transmitted through the sample, FTIR spectrometer can be used to acquire spectrum of light emitted by the sample. Such emission could be induced by various processes, and the most common ones are luminescence and Raman scattering. Little modification is required to an absorption FTIR spectrometer to record emission spectra and therefore many commercial FTIR spectrometers combine both absorption and emission/Raman modes.[13]
- Photocurrent spectra. This mode uses a standard, absorption FTIR spectrometer. The studied sample is placed instead of the FTIR detector, and its photocurrent, induced by the spectrometer's broadband source, is used to record the interferrogram, which is then converted into the photoconductivity spectrum of the sample.[14]
References
- ↑ 1.0 1.1 Griffiths, P.; de Hasseth, J.A. (18 May 2007). Fourier Transform Infrared Spectrometry (2nd ed.). Wiley-Blackwell. ISBN 0-471-19404-2.
- ↑ "The Infracord double-beam spectrophotometer". Clinical Science 16 (2). 1957.
- ↑ {http://books.google.co.in/books?id=ZecrNiUkHToC&pg=PA100&lpg=PA100&dq=Digilab+%2B+first+commercial+FTIR&source=bl&ots=5kaX5xpGX4&sig=mc8ClC39zazR6_5_wNI6wXCx9KE&hl=en&sa=X&ei=4Co2UcrqHMnEtAb50IAQ&ved=0CFkQ6AEwCTgK#v=onepage&q=Digilab%20%2B%20first%20commercial%20FTIR&f=false}
- ↑ {http://www.agilent.com/about/newsroom/presrel/varian/2004/15sep-v04044.htm}
- ↑ {http://www.agilent.co.in/about/newsroom/presrel/2009/27jul-gp09016.html}
- ↑ Banwell, C.N.; McCash, E.M. (1994). Fundamentals of Molecular Spectroscopy (4th ed.). McGraw-Hill. ISBN 0-07-707976-0.
- ↑ 7.0 7.1 7.2 7.3 7.4 Robert White (1990). Chromatography/Fourier transform infrared spectroscopy and its applications. Marcel Dekker. ISBN 0-8247-8191-0.
- ↑ Connes, J.; Connes, P. (1966). "Near-Infrared Planetary Spectra by Fourier Spectroscopy. I. Instruments and Results". Journal of the Optical Society of America 56 (7): 896–910. doi:10.1364/JOSA.56.000896.
- ↑ Chamberain, J.; Gibbs,J.E.; Gebbie, H.E. (1969). "The determination of refractive index spectra by fourier spectrometry". Infrared Physics 9 (4): 189–209. Bibcode:1969InfPh...9..185C. doi:10.1016/0020-0891(69)90023-2.
- ↑ Nishikida, K.; Nishio, E.; Hannah, R.W. (1995). Selected applications of FT-IR techniques. Gordon and Breach. p. 240. ISBN 2-88449-073-6.
- ↑ Beauchaine, J.P.; Peterman, J.W.; Rosenthal,R.J. (1988). "Applications of FT-IR/microscopy in forensic analysis". Microchimica Acta 94 (1-6): 133–138. doi:10.1007/BF01205855.
- ↑ Prati, S.; Joseph, E.; Sciutto, G.; Mazzeo, R. (2010). "New Advances in the Application of FTIR Microscopy and Spectroscopy for the Characterization of Artistic Materials". Acc. Chem. Res. 43 (6): 792–801. doi:10.1021/ar900274f. PMID 20476733.
- ↑ Michael Gaft, Renata Reisfeld, Gérard Panczer (2005). Luminescence spectroscopy of minerals and materials. Springer. p. 263. ISBN 3-540-21918-8.
- ↑ Jef Poortmans, Vladimir Arkhipov (2006). Thin film solar cells: fabrication, characterization and applications. John Wiley and Sons. p. 189. ISBN 0-470-09126-6.
External links
- Infracord spectrometer photograph
- The Grubb-Parsons-NPL cube interferometer Spectroscopy, part 2 by Dudley Williams, page 81
- infrared materials Properties of many salt crystals and useful links.
| ||||||||||||||||||||||||||