Fourier shell correlation
In structural biology, the three-dimensional Fourier shell correlation (FSC) measures the normalised cross-correlation coefficient between two 3-dimensional volumes over corresponding shells in Fourier space (i.e., as a function of spatial frequency[1]). The FSC is the three-dimensional extension of the two-dimensional Fourier ring correlation (FRC);[2] also known as: spatial frequency correlation function.[3]
Calculation
where 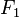 is the complex structure Factor for volume 1,
is the complex structure Factor for volume 1, 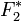 is the complex conjugate of the structure Factor for volume 2, and
is the complex conjugate of the structure Factor for volume 2, and 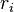 is the individual voxel element at radius
is the individual voxel element at radius  .[4][5][6] In this form, the FSC takes two three-dimensional data sets and converts them into a one-dimensional array.
.[4][5][6] In this form, the FSC takes two three-dimensional data sets and converts them into a one-dimensional array.
The FSC originated in cryo-electron microscopy and gradually proliferated to other fields. To measure the FSC, two independently determined 3D volumes are required. In cryo-electron microscopy, the two volumes are the result of two three-dimensional reconstructions, each based on half of the available data set. Typically, the even particle images form one half and the odd particles the other half of the data set. Most publications quote the FSC 0.5 resolution cutoff, which ad hoc criterion refers to when the correlation coefficient of the Fourier shells is equal to 0.5.[7][8] However, determining the resolution threshold remains a controversial issue: fixed-value thresholds were argued to be based on incorrect statistical assumptions.[6] Many other criteria using the FSC curve exist, including 3-σ criterion, 5-σ criterion, and the 0.143 cutoff. The half-bit criterion indicates at which resolution we have collected enough information to reliably interpret the 3-dimensional volume, and the (modified) 3-sigma criterion indicates where the FSC systematically emerges above the expected random correlations of the background noise.[6]
See also
Notes
- ↑ Harauz & van Heel, 1986
- ↑ van Heel, 1982
- ↑ Saxton & Baumeister, 1982
- ↑ "Image Science's FSC: Program to calculate the Fourier Shell Correlation (FSC) of two 3D volumes". fsc. Image Science. Retrieved 2009-04-09.
- ↑ "RF 3 - Phase Residual & Fourier shell correlation". SPIDER. Wadsworth Center. Retrieved 2009-04-09.
- ↑ 6.0 6.1 6.2 van Heel & Schatz, 2005
- ↑ Böttcher et al., 1997
- ↑ Frank, 2006, p250-251
References
- Harauz, G.; M. van Heel (1986). "Exact filters for general geometry three dimensional reconstruction". Optik 73: 146–156.
- van Heel, M.; Keegstra, W.; Schutter, W.; van Bruggen E.F.J. (1982). Arthropod hemocyanin studies by image analysis, in: Structure and Function of Invertebrate Respiratory Proteins,EMBO Workshop 1982, E.J. Wood. Life Chemistry Reports. Suppl. 1. pp. 69–73. ISBN 9783718601554.
- Saxton, W.O.; W. Baumeister (1982). "The correlation averaging of a regularly arranged bacterial cell envelope protein". J Microscopy 127 (2): 127–138. doi:10.1111/j.1365-2818.1982.tb00405.x.
- Böttcher, B.; Wynne, S.A.; Crowther, R.A. (1997). "Determination of the fold of the core protein of hepatitis B virus by electron microscopy". Nature 386 (6620): 88–91. doi:10.1038/386088a0. PMID 9052786.
- Frank, Joachim (2006). Three-Dimensional Electron Microscopy of Macromolecular Assemblies. New York: Oxford University Press. ISBN 0-19-518218-9.
- van Heel, M.; Schatz, M. (2005). "Fourier shell correlation threshold criteria". Journal of Structural Biology 151 (3): 250–262. doi:10.1016/j.jsb.2005.05.009. PMID 16125414.
External links
- EMstats Trends and distributions of maps in EM Data Bank (EMDB), e.g. resolution trends
![FSC(r) =
\frac{\displaystyle\sum_{r_i \in r}{ F_1(r_i) \cdot F_2(r_i)^{\ast} }}
{\displaystyle\sqrt[2]{\sum_{r_i \in r}{ \left|F_1(r_i)\right|^2} \cdot \sum_{r_i \in r}{\left|F_2(r_i)\right|^2}}}](../I/m/837bd058129bfdb9808b5d60f044f8a0.png)