Forbidden mechanism
In physics, a forbidden mechanism or forbidden line is a spectral line emitted by atomic nuclei, atoms, or molecules undergoing nominally "forbidden" energy transitions not normally "allowed" by the selection rules of quantum mechanics. In formal physics, this means that the process cannot proceed via the most efficient (electric dipole) route.
Although the transitions are nominally "forbidden", there is a small probability of their spontaneous occurrence, should an atomic nucleus, atom or molecule be raised to an excited state. More precisely, there is a certain probability that such an excited entity will make a forbidden transition to a lower energy state per unit time; by definition, this probability is much lower than that for any transition permitted or allowed by the selection rules. Therefore, if a state can de-excite via a permitted transition (or otherwise, e.g. via collisions) it will almost certainly do so rather than choosing the forbidden route. Nevertheless, most "forbidden" transitions are only relatively unlikely: states that can only decay in this way (so-called meta-stable states) usually have lifetimes of order milliseconds to seconds, compared to less than a microsecond for decay via permitted transitions. In some radioactive decay systems, multiple levels of "forbiddenness" can stretch life times by many orders of magnitude for each additional unit by which the system changes beyond what is most allowed under the selection rules. Such excited states can last years, or even for many billions of years (too long to have been measured).
In radioactive decay
Gamma decay
The most common mechanism for suppression of the rate of gamma decay of excited atomic nuclei, and thus make possible the existence of a metastable isomer for the nucleus, is lack of a decay route for the excited state that will change nuclear angular momentum (along any given direction) by the most common ("allowed") amount of 1 quantum unit 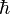 of spin angular momentum. Such a change is necessary to emit a gamma photon, which has a spin of 1 unit in this system. Integral changes of 2,3,4, and more units in angular momentum are possible (the emitted photons carry off the additional angular momentum), but changes of more than 1 unit are known as forbidden transitions. Each degree of "forbiddeness" (additional unit of spin change larger than 1, that the emitted gamma ray must carry) inhibits decay rate by about 5 orders of magnitude.[1] The highest known spin change of 8 units occurs in the decay of Ta-180m, which suppresses its decay by a factor of 1035 from that associated with 1 unit, so that instead of a natural gamma decay half life of 10−12 seconds, it has a half life of more than 1023 seconds, or at least 3 x 1015 years, and thus has yet to be observed to decay.
of spin angular momentum. Such a change is necessary to emit a gamma photon, which has a spin of 1 unit in this system. Integral changes of 2,3,4, and more units in angular momentum are possible (the emitted photons carry off the additional angular momentum), but changes of more than 1 unit are known as forbidden transitions. Each degree of "forbiddeness" (additional unit of spin change larger than 1, that the emitted gamma ray must carry) inhibits decay rate by about 5 orders of magnitude.[1] The highest known spin change of 8 units occurs in the decay of Ta-180m, which suppresses its decay by a factor of 1035 from that associated with 1 unit, so that instead of a natural gamma decay half life of 10−12 seconds, it has a half life of more than 1023 seconds, or at least 3 x 1015 years, and thus has yet to be observed to decay.
Although gamma decays with nuclear angular momentum changes of 2, 3, 4, etc, are "forbidden", they are only relatively forbidden, and do proceed, but with a slower rate than the normal "allowed" change of 1 unit. However, gamma emission is "absolutely forbidden" when the nucleus begins in a zero-spin state, as such an emission would not conserve angular momentum. These transitions cannot occur by gamma decay, but must proceed by another route, such as beta decay in some cases, or internal conversion where beta decay is not favored.
Beta decay
Beta decays here are classified according to the L-value of the emitted radiation. Unlike gamma decays, beta decays may proceed from a nucleus with a spin of zero and even parity, to a nucleus also with a spin of zero and even parity ("Fermi transition"). This is possible because the electron and neutrino emitted may be of opposing spin (giving a radiation total angular momentum of zero), thus preserving angular momentum of the initial state even if the nucleus remains at spin-zero before and after emission. This type of emission is "super-allowed" meaning that it is the most rapid type of beta decay in nuclei that are susceptible to a change in proton/neutron ratios that accompanies a beta decay process.
The next possible total angular momentum of the electron and neutrino emitted in beta decay is a combined spin of 1 (electron and neutrino spinning in the same direction), and is "allowed". This type of emission ("Gamow-Teller transition") changes nuclear spin by a unit of 1 to compensate. States involving higher angular momenta of the emitted radiation (2, 3, 4, etc.) are "forbidden", and are ranked in degree of forbiddenness by their increasing angular momentum.
Specifically, when L > 0, the decay is referred to as "forbidden". Nuclear selection rules require L-values greater than two to be accompanied by changes in both nuclear spin (J) and parity (π). The selection rules for the Lth forbidden transitions are:
where Δπ = 1 or −1 corresponds to no parity change or parity change, respectively. As noted, the special case of a "Fermi" 0+ → 0+ transition (which in gamma decay is absolutely forbidden) is referred to as "super-allowed" for beta decay, and proceeds very quickly if beta decay is possible. The following table lists the ΔJ and Δπ values for the first few values of L:
| Forbiddenness | ΔJ | Δπ |
|---|---|---|
| Superallowed | 0+ → 0+ | no |
| Allowed | 0, 1 | no |
| First forbidden | 0, 1, 2 | yes |
| Second forbidden | 1, 2, 3 | no |
| Third forbidden | 2, 3, 4 | yes |
As with gamma decay, each degree of increasing forbiddenness increases the half life of the beta decay process involved by a factor of about 4 to 5 orders of magnitude.[2]
In solid-state physics
Forbidden transitions in rare earth atoms such as erbium and neodymium make them useful as dopants for solid-state lasing media. In such media, the atoms are held in a matrix which keeps them from de-exciting by collision, and the long half life of their excited states makes them easy to "optically pump" to create a large population of excited atoms. Neodymium doped glass derives its unusual coloration from "forbidden" f-f transitions within the neodymium atom, and is used in extremely high power solid state lasers.
In astrophysics
Forbidden emission lines have only been observed in extremely low-density gases and plasmas, either in outer space or in the extreme upper atmosphere of the Earth. Even the hardest laboratory vacuum on Earth is still too dense for forbidden line emission to occur before atoms are collisionally de-excited. However, in space environments, densities may be only a few atoms per cubic centimetre, making atomic collisions unlikely. Under such conditions, once an atom or molecule has been excited for any reason into a meta-stable state, then it is almost certain to decay by emitting a forbidden-line photon. Since meta-stable states are rather common, forbidden transitions account for a significant percentage of the photons emitted by the ultra-low density gas in space.
Forbidden lines of nitrogen ([N II] at 654.8 and 658.4 nm), sulfur ([S II] at 671.6 and 673.1 nm), and oxygen ([O II] at 372.7 nm, and [O III] at 495.9 and 500.7 nm) are commonly observed in astrophysical plasmas. These lines are important to the energy balance of such things as planetary nebulae and H II regions. The forbidden 21-cm hydrogen line is particularly important for radio astronomy as it allows very cold neutral hydrogen gas to be seen.
Notation
Forbidden line transitions are noted by placing square brackets around the atomic or molecular species in question, e.g. [O III] or [S II].
References
- Osterbrock, D.E., Astrophysics of gaseous nebulae and active galactic nuclei, University Science Books, 1989, ISBN 0-935702-22-9.