Fluprednisolone
Fluprednisolone
 |
 |
| Names |
| IUPAC name
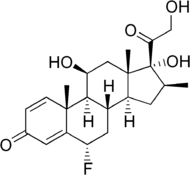
(6S,8S,9S,10R,11S,13S,14S,17R)-6-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-one |
| Identifiers |
| |
53-34-9  Y Y |
| ChEMBL |
ChEMBL1200774  N N |
| Jmol-3D images |
Image |
| KEGG |
D04227  Y Y |
| PubChem |
5876 |
C[C@]12C[C@@H]([C@H]3[C@H]([C@@H]1CC[C@@]2(C(=O)CO)O)C[C@@H](C4=CC(=O)C=C[C@]34C)F)O
|
| Properties |
| |
C21H27FO5 |
| Molar mass |
378.43 g/mol |
Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) |
 N verify (what is: N verify (what is:  Y/ Y/ N?) N?) |
| Infobox references |
|
|
Fluprednisolone is a pregnane. It is a corticosteroid.[1]
References
- ↑ Naggar VF, Gouda MW, Khalil SA (December 1977). "In vitro adsorption of some corticosteroids on antacids". Pharmazie 32 (12): 778–81. PMID 613316.
|
|---|
| | Agonists | |
|---|
| Antagonists /
SGRMs | |
|---|
| | Synthesis modifiers | |
|---|
| |
| | |
|---|
| | Description |
- Glands
- Hormones
- thyroid
- mineralocorticoids
- Physiology
- Development
|
|---|
| | Disease |
- Diabetes
- Congenital
- Neoplasms and cancer
- Other
- Symptoms and signs
|
|---|
| | Treatment |
- Procedures
- Drugs
- calcium balance
- corticosteroids
- oral hypoglycemics
- pituitary and hypothalamic
- thyroid
|
|---|
|
|
|
|---|
| | Receptor | |
|---|
| | Enzyme | |
|---|
| | Others | |
|---|
| See also: Androgenics • Estrogenics • Mineralocorticoidics • Progestogenics |
|

