Fluorometer
_in_the_field..jpg)
A fluorometer or fluorimeter is a device used to measure parameters of fluorescence: its intensity and wavelength distribution of emission spectrum after excitation by a certain spectrum of light.[1] These parameters are used to identify the presence and the amount of specific molecules in a medium. Modern fluorometers are capable of detecting fluorescent molecule concentrations as low as 1 part per trillion.
Fluorescence analysis can be orders of magnitude more sensitive than other techniques. Applications include chemistry/biochemistry, medicine, environmental monitoring. For instance, they are used to measure chlorophyll fluorescence to investigate plant physiology.
Components and Design
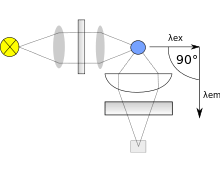
Typically fluorometers utilize a double beam. These two beams work in tandem to decrease the noise created from radiant power fluctuations. The upper beam is passed through a filter or monochromator and passes through the sample. The lower beam is passed through an attenuator and adjusted to try and match the fluorescent power given off from the sample. Light from the fluorescence of the sample and the lower, attenuated beam are detected by separate transducers and converted to an electrical signal that is interpreted by a computer system.
Within the machine the transducer that detects fluorescence created from the upper beam is located a distance away from the sample and at a 90-degree angle from the incident, upper beam. The machine is constructed like this to decrease the stray light from the upper beam that may strike the detector. The optimal angle is 90 degrees. There are two different approaches to handling the selection of incident light that gives way to different types fluorometers. If filters are used to select wavelengths of light, the machine is called a fluorometer. While a spectrofluorometer will typically use two monochromators. However, some spectrofluorometers may use one filter and one monochromator, they are still called a spectrofluorometer.
Sources for fluorometers are often dependent on the type of sample being tested. Among the most common source for fluorometers is the low-pressure mercury lamp. This provides many excitation wavelengths, making it the most versatile. However, this lamp is not a continuous source of radiation. The xenon arc lamp is used when a continuous source of radiation is needed. Both of these sources provide a suitable spectrum of ultraviolet light that induces chemiluminescence. These are just two of the many possible light sources.
Glass and silica cells are often the vessels in which the sample is placed. The scientist must be very careful to not leave fingerprints or any other sort of mark on the outside of the cell.
Uses
Fluorimetry is widely used by the dairy industry to verify whether pasteurization has been successful. This is done using a reagent which is hydrolysed to a fluorophore and phosphoric acid by alkaline phosphatase in milk.[2] If pasteurization has been successful then alkaline phosphatase will be entirely denatured and the sample will not fluoresce. This works because pathogens in milk are killed by any heat treatment which denatures alkaline phosphatase.[3][4]
Fluorophos assays are required by milk producers in the UK to prove successful pasteurization has occurred,[5] so all UK dairies contain fluorimetry equipment.
Fluorometer types
There are two basic types of fluorometers, the filter fluorometer and the spectrofluorometer. The difference between them is the way they select the wavelengths of incident light. A filter fluorometer will use filters while a spectrofluorometer will use grating monochromators. Filter fluorometers are often purchased/built at a lower cost but are less sensitive and less resolute than spectrofluorometers.
See also
- Fluorescence spectroscopy, for a fuller discussion of instrumentation
- Chlorophyll fluorescence, to investigate plant ecophysiology.
- Integrated fluorometer to measure gas exchange and chlorophyll fluorescence of leaves.
- Radiometer, to measure various electromagnetic radiation
- Spectrometer, to analyze spectrum of electromagnetic radiation
- Scatterometer, to measure scattered radiation
- Microfluorimetry, to measure fluorescence on a microscopic level
References
- ↑ "Fluorescence Spectrophotometry". Encyclopedia of Life Sciences. Macmillan Publishers Ltd. 2002.
- ↑ Langridge, E W. The Determination of Phosphatase Activity. Quality Management Ltd. Retrieved 2013-12-20.
- ↑ Kay, H. (1935). "Some Results of the Application of a Simple Test for Efficiency of Pasteurisation". The Lancet 225 (5835): 1516–1518. doi:10.1016/S0140-6736(01)12532-8.
- ↑ Hoy, W. A.; Neave, F. K. (1937). "The Phosphatase Test for Efficient Pasteurisation". The Lancet 230 (5949): 595. doi:10.1016/S0140-6736(00)83378-4.
- ↑ BS EN ISO 11816-1:2013