Fluorescence anisotropy
Fluorescence anisotropy is the phenomenon where the light emitted by a fluorophore has unequal intensities along different axes of polarization. Early pioneers in the field include Aleksander Jablonski, Gregorio Weber,[1] and Andreas Albrecht.[2] The principles of fluorescence polarization and some applications of the method are presented in Lakowicz's book.[3]
Principle
In fluorescence, a molecule absorbs a photon and gets excited to a higher energy state. After a short delay (the average represented as the fluorescence lifetime 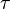 ), it comes down to a lower state by losing some of the energy as heat and emitting the rest of the energy as another photon. The excitation and de-excitation involve the redistribution of electrons about the molecule. Hence, excitation by a photon can occur only if the electric field of the light is oriented in a particular axis about the molecule. Also, the emitted photon will have a specific polarization with respect to the molecule.
), it comes down to a lower state by losing some of the energy as heat and emitting the rest of the energy as another photon. The excitation and de-excitation involve the redistribution of electrons about the molecule. Hence, excitation by a photon can occur only if the electric field of the light is oriented in a particular axis about the molecule. Also, the emitted photon will have a specific polarization with respect to the molecule.
When polarized light is applied to a group of randomly oriented fluorophores, most of the excited molecules will be those oriented within a particular range of angles to the applied polarization. If they do not move, the emitted light will also be polarized within a particular range angles to the applied light. This intrinsic anisotropy (denoted r0) is usually measured by embedding the fluorophore in a frozen polyol.
When the fluorophores can freely change their orientation before re-emitting the photons, the degree of polarization of the emitted light will be reduced. The degree of decorrelation in the polarization of the incident and emitted light depends on how quickly the fluorophore orientation gets scrambled ( the rotational lifetime 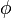 ) compared to the fluorescence lifetime (
) compared to the fluorescence lifetime (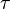 ). The scrambling of orientations can occur by the whole molecule tumbling or by the rotation of only the fluorescent part. The rate of tumbling is related to the measured anisotropy by the relationship:
). The scrambling of orientations can occur by the whole molecule tumbling or by the rotation of only the fluorescent part. The rate of tumbling is related to the measured anisotropy by the relationship:
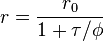
Where r is the observed anisotropy, r0 is the intrinsic anisotropy of the molecule, 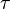 is the fluorescence lifetime and
is the fluorescence lifetime and 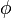 is the rotational time constant.[4]
is the rotational time constant.[4]
This analysis is valid only if the fluorophores are relatively far apart. If they are very close to another, they can exchange energy by FRET and because the emission can occur from one of many independently moving (or oriented) molecules this results in a lower than expected anisotropy or a greater decorrelation. This type of homotransfer Förster resonance energy transfer is called energy migration FRET or emFRET
Applications
Fluorescence anisotropy can be used to measure the binding constants and kinetics of reactions that cause a change in the rotational time of the molecules. If the fluorophore is bound to a small molecule, the rate at which it tumbles can decrease significantly when it is bound tightly to a large protein. If the fluorophore is attached to the larger protein in a binding pair, the difference in polarization between bound and unbound states will be smaller (because the unbound protein will already be fairly stable and tumble slowly to begin with) and the measurement will be less accurate. The degree of binding is calculated by using the difference in anisotropy of the partially bound, free and fully bound (large excess of protein) states measured by titrating the two binding partners.
If the fluorophore is bound to a relatively large molecule like a protein or an RNA, the change in the mobility accompanying folding can be used to study the dynamics of folding. This provides a measure of the dynamics of how the protein achieves its final, stable 3D shape.
Fluorescence anisotropy is also applied to microscopy, with use of polarizers in the path of the illuminating light and also before the camera. This can be used to study the local viscosity of the cytosol or membranes, with the latter giving information about the membrane microstructure and the relative concentrations of various lipids. This technique has also been used to detect the binding of molecules to their partners in signaling cascades in response to certain cues.
The phenomenon of emFRET and the associated decrease in anisotropy when close interactions occur between fluorophores has been used to study the aggregation of proteins in response to signaling.
See also
References
- ↑ Weber, G., 1953. Rotational Brownian motion and polarization of the fluorescence of solutions. Adv. Protein Chem. 8:415-459
- ↑ Albrecht, A., 1961. Polarizations and assignments of transitions: the method of photoselection. J. Mol. Spectrosc. 6:84-108.
- ↑ Lakowicz, J.R., 2006. Principles of Fluorescence Spectroscopy (3rd ed., Springer. Chapter 10-12 deal with fluorescence polarization spectroscopy.)
- ↑ Valeur, Bernard. 2001. Molecular Fluorescence: Principles and Applications Wiley-VCH, p.29
| ||||||||||||||||||||||||||||||||||||||