Fish fin
.png)
(1) pectoral fins (paired), (2) pelvic fins (paired), (3) dorsal fin,
(4) adipose fin, (5) anal fin, (6) caudal (tail) fin
Fish fins are the most distinctive features of a fish, composed of bony spines protruding from the body with skin covering them and joining them together, either in a webbed fashion, as seen in most bony fish, or similar to a flipper, as seen in sharks. Apart from the tail or caudal fin, fins have no direct connection with the spine and are supported by muscles only. Their principal function is to help the fish swim. Fins located in different places on the fish serve different purposes, such as moving forward, turning, keeping an upright position or stopping. Most fish use fins when swimming, flying fish use pectoral fins for gliding, and frogfish use them for crawling. Fins can also be used for other purposes; male sharks and mosquitofish use a modified fin to deliver sperm, thresher sharks use their tail fin to stun prey, reef stonefish have spines in their dorsal fins that inject venom, anglerfish use the first spine of their dorsal fin like a fishing rod to lure prey, and triggerfish avoid predators by squeezing into coral crevices and using spines in their fins to lock themselves in place.
Types
For every type of fin, there are a number of fish species in which this particular fin has been lost during evolution.
| Pectoral fins | |
The paired pectoral fins are located on each side, usually just behind the operculum, and are homologous to the forelimbs of tetrapods.
|
|---|---|---|
| Pelvic fins (Ventral fins) |
 |
The paired pelvic or ventral fins are typically located ventrally below and behind the pectoral fins, although in many fish families they may be positioned in front of the pectoral fins (e.g. cods). They are homologous to the hindlimbs of tetrapods. The pelvic fin assists the fish in going up or down through the water, turning sharply, and stopping quickly. |
| Dorsal fin |  |
 Dorsal fin of a chub (Leuciscus cephalus) Dorsal fins are located on the back. A fish can have up to three dorsal fins. The dorsal fins serve to protect the fish against rolling, and assist it in sudden turns and stops.
|
| Anal fin |  |
The anal fin is located on the ventral surface behind the anus. This fin is used to stabilize the fish while swimming. |
| Adipose fin |  |
The adipose fin is a soft, fleshy fin found on the back behind the dorsal fin and just forward of the caudal fin. It is absent in many fish families, but is found in Salmonidae, characins and catfishes. Its function has remained a mystery, and is frequently clipped off to mark hatchery-raised fish, though data from 2005 showed that trout with their adipose fin removed have an 8% higher tailbeat frequency.[2][3] Additional information released in 2011 has suggested that the fin may be vital for the detection of, and response to, stimuli such as touch, sound and changes in pressure. Canadian researchers identified a neural network in the fin, indicating that it likely has a sensory function, but are still not sure exactly what the consequences of removing it are.[4][5]
Research published in 2014 indicates that the adipose fin has evolved repeatedly in separate lineages.[6] |
| Caudal fin (Tail fin) |
  |
The caudal fin is the tail fin (from the Latin cauda meaning tail), located at the end of the caudal peduncle and is used for propulsion. See body-caudal fin locomotion.
(A) - Heterocercal means the vertebrae extend into the upper lobe of the tail, making it longer (as in sharks).
(B) - Protocercal means the vertebrae extend to the tip of the tail and the tail is symmetrical but not expanded (as in amphioxus) (C) - Homocercal where the fin appears superficially symmetric but in fact the vertebrae extend for a very short distance into the upper lobe of the fin (D) - Diphycercal means the vertebrae extend to the tip of the tail and the tail is symmetrical and expanded (as in the bichir, lungfish, lamprey and coelacanth). Most Palaeozoic fishes had a diphycercal heterocercal tail.[7] Most modern fishes have a homocercal tail. These appear in a variety of shapes, and can appear:
|
| Caudal keel Finlets |
|
Some types of fast-swimming fish have a horizontal caudal keel just forward of the tail fin. Much like the keel of a ship, this is a lateral ridge on the caudal peduncle, usually composed of scutes (see below), that provides stability and support to the caudal fin. There may be a single paired keel, one on each side, or two pairs above and below.
Finlets are small fins, generally behind the dorsal and anal fins (in bichirs, there are only finlets on the dorsal surface and no dorsal fin). In some fish such as tuna or sauries, they are rayless, non-retractable, and found between the last dorsal and/or anal fin and the caudal fin. |
Bony fishes
Bony fishes form a taxonomic group called Osteichthyes. They have skeletons made of bone, and can be contrasted with cartilaginous fishes which have skeletons made of cartilage. Bony fishes are divided into ray-finned and lobe-finned fish. Most fish are ray-finned, an extremely diverse and abundant group consisting of over 30,000 species. It is the largest class of vertebrates in existence today. In the distant past, lobe-finned fish were abundant. Nowadays they are mainly extinct, with only eight living species. Bony fish have fin spines and rays called lepidotrichia. They typically have swim bladders, which allows the fish to create a neutral balance between sinking and floating without having to use its fins. However, these are absent in many species, and have developed into primitive lungs in the lungfishes. Bony fishes also have an operculum, which helps them breathe without having to use fins to swim.
Lobe-finned

Lobe-finned fishes are a class of bony fishes called Sarcopterygii. They have fleshy, lobed, paired fins, which are joined to the body by a single bone.[8] The fins of lobe-finned fish differ from those of all other fish in that each is borne on a fleshy, lobelike, scaly stalk extending from the body. Pectoral and pelvic fins have articulations resembling those of tetrapod limbs. These fins evolved into legs of the first tetrapod land vertebrates, amphibians. They also possess two dorsal fins with separate bases, as opposed to the single dorsal fin of ray-finned fish.
The coelacanth is another lobe-finned fish which is still extant. It is thought to have evolved into roughly its current form about 408 million years ago, during the early Devonian.[9] It has not essentially evolved further from its ancient form, and it is regarded as a living fossil.[10] Locomotion of the coelacanths is unique to their kind. To move around, coelacanths most commonly take advantage of up or downwellings of the current and drift. They use their paired fins to stabilize their movement through the water. While on the ocean floor their paired fins are not used for any kind of movement. Coelacanths can create thrust for quick starts by using their caudal fins. Due to the high number of fins it possesses, the coelacanth has high maneuverability and can orient their bodies in almost any direction in the water. They have been seen doing headstands and swimming belly up. It is thought that their rostral organ helps give the coelacanth electroperception, which aids in their movement around obstacles.[11]
Ray-finned
Ray-finned fishes are a class of bony fishes called Actinopterygii. Their fins contain spines or rays. A fin may contain only spiny rays, only soft rays, or a combination of both. If both are present, the spiny rays are always anterior. Spines are generally stiff and sharp. Rays are generally soft, flexible, segmented, and may be branched. This segmentation of rays is the main difference that separates them from spines; spines may be flexible in certain species, but they will never be segmented.
Spines have a variety of uses. In catfish, they are used as a form of defense; many catfish have the ability to lock their spines outwards. Triggerfish also use spines to lock themselves in crevices to prevent them being pulled out.
Lepidotrichia are bony, bilaterally paired, segmented fin rays found in bony fishes. They develop around actinotrichia as part of the dermal exoskeleton.[12] Lepidotrichia may have some cartilage or bone in them as well. They are segmented and appear as a series of disks stacked one on top of another. The genetic basis for the formation of the fin rays is thought to be genes coded for the production of certain proteins. It has been suggested that the evolution of the tetrapod limb from lobe-finned fishes is related to the loss of these proteins.[13]
Cartilaginous fishes

Cartilaginous fishes are a class of fishes called Chondrichthyes. They have skeletons made of cartilage rather than bone. The class includes sharks, rays and chimaeras. Shark fin skeletons are elongated and supported with soft and unsegmented rays named ceratotrichia, filaments of elastic protein resembling the horny keratin in hair and feathers.[14] Originally the pectoral and pelvic girdles, which do not contain any dermal elements, did not connect. In later forms, each pair of fins became ventrally connected in the middle when scapulocoracoid and pubioischiadic bars evolved. In rays, the pectoral fins have connected to the head and are very flexible. One of the primary characteristics present in most sharks is the heterocercal tail, which aids in locomotion.[15] Most sharks have eight fins. Sharks can only drift away from objects directly in front of them because their fins do not allow them to move in the tail-first direction.[16]
As with most fish, the tails of sharks provide thrust, making speed and acceleration dependent on tail shape. Caudal fin shapes vary considerably between shark species, due to their evolution in separate environments. Sharks possess a heterocercal caudal fin in which the dorsal portion is usually noticeably larger than the ventral portion. This is because the shark's vertebral column extends into that dorsal portion, providing a greater surface area for muscle attachment. This allows more efficient locomotion among these negatively buoyant cartilaginous fish. By contrast, most bony fish possess a homocercal caudal fin.[17]
Tiger sharks have a large upper lobe, which allows for slow cruising and sudden bursts of speed. The tiger shark must be able to twist and turn in the water easily when hunting to support its varied diet, whereas the porbeagle shark, which hunts schooling fish such as mackerel and herring, has a large lower lobe to help it keep pace with its fast-swimming prey.[18] Other tail adaptations help sharks catch prey more directly, such as the thresher shark's usage of its powerful, elongated upper lobe to stun fish and squid.
Generating thrust
Foil shaped fins generate thrust when moved, the lift of the fin sets water or air in motion and pushes the fin in the opposite direction. Aquatic animals get significant thrust by moving fins back and forth in water. Often the tail fin is used, but some aquatic animals generate thrust from pectoral fins.[19]


Finlets may influence the way a vortex develops around the tail fin.
Cavitation occurs when negative pressure causes bubbles (cavities) to form in a liquid, which then promptly and violently collapse. It can cause significant damage and wear.[20] Cavitation damage can occur to the tail fins of powerful swimming marine animals, such as dolphins and tuna. Cavitation is more likely to occur near the surface of the ocean, where the ambient water pressure is relatively low. Even if they have the power to swim faster, dolphins may have to restrict their speed because collapsing cavitation bubbles on their tail are too painful.[21] Cavitation also slows tuna, but for a different reason. Unlike dolphins, these fish do not feel the bubbles, because they have bony fins without nerve endings. Nevertheless, they cannot swim faster because the cavitation bubbles create a vapor film around their fins that limits their speed. Lesions have been found on tuna that are consistent with cavitation damage.[21]
Scombrid fishes (tuna, mackerel and bonito) are particularly high-performance swimmers. Along the margin at the rear of their bodies is a line of small rayless, non-retractable fins, known as finlets. There has been much speculation about the function of these finlets. Research done in 2000 and 2001 by Nauen and Lauder indicated that "the finlets have a hydrodynamic effect on local flow during steady swimming" and that "the most posterior finlet is oriented to redirect flow into the developing tail vortex, which may increase thrust produced by the tail of swimming mackerel".[22][23][24]
Fish use multiple fins, so it is possible that a given fin can have a hydrodynamic interaction with another fin. In particular, the fins immediately upstream of the caudal (tail) fin may be proximate fins that can directly affect the flow dynamics at the caudal fin. In 2011, researchers using volumetric imaging techniques were able to generate "the first instantaneous three-dimensional views of wake structures as they are produced by freely swimming fishes". They found that "continuous tail beats resulted in the formation of a linked chain of vortex rings" and that "the dorsal and anal fin wakes are rapidly entrained by the caudal fin wake, approximately within the timeframe of a subsequent tail beat".[25]
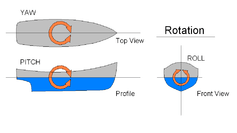
Controlling motion
Once motion has been established, the motion itself can be controlled with the use of other fins.[19][26]

.jpg)
The bodies of reef fishes are often shaped differently from open water fishes. Open water fishes are usually built for speed, streamlined like torpedoes to minimise friction as they move through the water. Reef fish operate in the relatively confined spaces and complex underwater landscapes of coral reefs. For this manoeuvrability is more important than straight line speed, so coral reef fish have developed bodies which optimize their ability to dart and change direction. They outwit predators by dodging into fissures in the reef or playing hide and seek around coral heads.[30] The pectoral and pelvic fins of many reef fish, such as butterflyfish, damselfish and angelfish, have evolved so they can act as brakes and allow complex manoeuvres.[32] Many reef fish, such as butterflyfish, damselfish and angelfish, have evolved bodies which are deep and laterally compressed like a pancake, and will fit into fissures in rocks. Their pelvic and pectoral fins are designed differently, so they act together with the flattened body to optimise manoeuvrability.[30] Some fishes, such as puffer fish, filefish and trunkfish, rely on pectoral fins for swimming and hardly use tail fins at all.[32]
Reproduction

Male cartilaginous fishes (sharks and rays), as well as the males of some live-bearing ray finned fishes, have fins that have been modified to function as intromittent organs, reproductive appendages which allow internal fertilization. In ray finned fish they are called gonopodia or andropodia, and in cartilaginous fish they are called claspers.
Gonopodia are found on the males of some species in the Anablepidae and Poeciliidae families. They are anal fins that have been modified to function as movable intromittent organs and are used to impregnate females with milt during mating. The third, fourth and fifth rays of the male's anal fin are formed into a tube-like structure in which the sperm of the fish is ejected.[35] When ready for mating, the gonopodium becomes erect and points forward towards the female. The male shortly inserts the organ into the sex opening of the female, with hook-like adaptations that allow the fish to grip onto the female to ensure impregnation. If a female remains stationary and her partner contacts her vent with his gonopodium, she is fertilized. The sperm is preserved in the female's oviduct. This allows females to fertilize themselves at any time without further assistance from males. In some species, the gonopodium may be half the total body length. Occasionally the fin is too long to be used, as in the "lyretail" breeds of Xiphophorus helleri. Hormone treated females may develop gonopodia. These are useless for breeding.
Similar organs with similar characteristics are found in other fishes, for example the andropodium in the Hemirhamphodon or in the Goodeidae.[36]
Claspers are found on the males of cartilaginous fishes. They are the posterior part of the pelvic fins that have also been modified to function as intromittent organs, and are used to channel semen into the female's cloaca during copulation. The act of mating in sharks usually includes raising one of the claspers to allow water into a siphon through a specific orifice. The clasper is then inserted into the cloaca, where it opens like an umbrella to anchor its position. The siphon then begins to contract expelling water and sperm.[37][38]
Other uses
The Indo-Pacific sailfish has a prominent dorsal fin. Like scombroids and other billfish, they streamline themselves by retracting their dorsal fins into a grove in their body when they swim.[39] The huge dorsal fin, or sail, of the sailfish is kept retracted most of the time. Sailfish raise them if they want to herd a school of small fish, and also after periods of high activity, presumably to cool down.[39][40]



The oriental flying gurnard has large pectoral fins which it normally holds against its body, and expands when threatened to scare predators. Despite its name, it is a demersal fish, not a flying fish, and uses its pelvic fins to walk along the bottom of the ocean.[42][43]
Fins can have an adaptive significance as sexual ornaments. During courtship, the female cichlid, Pelvicachromis taeniatus, displays a large and visually arresting purple pelvic fin. "The researchers found that males clearly preferred females with a larger pelvic fin and that pelvic fins grew in a more disproportionate way than other fins on female fish."[44][45]



Evolution
Aristotle recognised the distinction between analogous and homologous structures, and made the following prophetic comparison: "Birds in a way resemble fishes. For birds have their wings in the upper part of their bodies and fishes have two fins in the front part of their bodies. Birds have feet on their underpart and most fishes have a second pair of fins in their under-part and near their front fins."
There is an old theory, which has been often disregarded in science textbooks, "that fins and (later) limbs evolved from the gills of an extinct vertebrate". Gaps in the fossil record had not allowed a definitive conclusion. In 2009, researchers from the University of Chicago found evidence that the "genetic architecture of gills, fins and limbs is the same", and that "the skeleton of any appendage off the body of an animal is probably patterned by the developmental genetic program that we have traced back to formation of gills in sharks".[49][50][51]
Fish are the ancestors of all mammals, reptiles, birds and amphibians.[52] In particular, terrestrial tetrapods (four-legged animals) evolved from fish and made their first forays onto land 400 million years ago. They used paired pectoral and pelvic fins for locomotion. The pectoral fins developed into forelegs (arms in the case of humans) and the pelvic fins developed into hind legs.[53] Much of the genetic machinery that builds a walking limb in a tetrapod is already present in the swimming fin of a fish.[54][55]

In 2011, researchers at Monash University in Australia used primitive but still living lungfish "to trace the evolution of pelvic fin muscles to find out how the load-bearing hind limbs of the tetrapods evolved."[56][57] Further research at the University of Chicago found bottom-walking lungfishes had already evolved characteristics of the walking gaits of terrestrial tetrapods.[58][59]
In a classic example of convergent evolution, the pectoral limbs of pterosaurs, birds and bats further evolved along independent paths into flying wings. Even with flying wings there are many similarities with walking legs, and core aspects of the genetic blueprint of the pectoral fin have been retained.[60][61]
About 200 million years ago the first mammals appeared. Several groups of these mammals started returning to the sea, including the cetaceans (whales, dolphins and porpoises). Recent DNA analysis suggests that cetaceans evolved from within the even-toed ungulates, and that they share a common ancestor with the hippopotamus.[62][63] About 23 million years ago another group of bearlike land mammals started returning to the sea. These were the seals.[64] What had become walking limbs in cetaceans and seals evolved independently into new forms of swimming fins. The forelimbs became flippers, while the hindlimbs were either lost (cetaceans) or also modified into flipper (pinnipeds). In cetaceans, the tail gained two fins at the end, called a fluke.[65] Fish tails are usually vertical and move from side to side. Cetacean flukes are horizontal and move up and down, because cetacean spines bend the same way as in other mammals.[66][67]
Ichthyosaurs are ancient reptiles that resembled dolphins. They first appeared about 245 million years ago and disappeared about 90 million years ago.
"This sea-going reptile with terrestrial ancestors converged so strongly on fishes that it actually evolved a dorsal fin and tail fin for improved aquatic locomotion. These structures are all the more remarkable because they evolved from nothing — the ancestral terrestrial reptile had no hump on its back or blade on its tail to serve as a precursor."[68]
The biologist Stephen Jay Gould said the ichthyosaur was his favorite example of convergent evolution.[69]
Fins or flippers of varying forms and at varying locations (limbs, body, tail) have also evolved in a number of other tetrapod groups, including diving birds such as penguins (modified from wings), sea turtles (forelimbs modified into flippers), mosasaurs (limbs modified into flippers), and sea snakes (vertically expanded, flattened tail fin).
Robotic fins

|
| |
|
| |
|
| |
|
| |
|
|
The use of fins for the propulsion of aquatic animals can be remarkably effective. It has been calculated that some fish can achieve a propulsive efficiency greater than 90%.[19] Fish can accelerate and maneuver much more effectively than boats or submarine, and produce less water disturbance and noise. This has led to biomimetic studies of underwater robots which attempt to emulate the locomotion of aquatic animals.[70] An example is the Robot Tuna built by the Institute of Field Robotics, to analyze and mathematically model thunniform motion.[71] In 2005, the Sea Life London Aquarium displayed three robotic fish created by the computer science department at the University of Essex. The fish were designed to be autonomous, swimming around and avoiding obstacles like real fish. Their creator claimed that he was trying to combine "the speed of tuna, acceleration of a pike, and the navigating skills of an eel."[72][73][74]
The AquaPenguin, developed by Festo of Germany, copies the streamlined shape and propulsion by front flippers of penguins.[75][76] Festo also developed AquaRay,[77] AquaJelly[78] and AiraCuda,[79] respectively emulating the locomotion of manta rays, jellyfish and barracuda.
In 2004, Hugh Herr at MIT prototyped a biomechatronic robotic fish with a living actuator by surgically transplanting muscles from frog legs to the robot and then making the robot swim by pulsing the muscle fibers with electricity.[80][81]
Robotic fish offer some research advantages, such as the ability to examine an individual part of a fish design in isolation from the rest of the fish. However, this risks oversimplifying the biology so key aspects of the animal design are overlooked. Robotic fish also allow researchers to vary a single parameter, such as flexibility or a specific motion control. Researchers can directly measure forces, which is not easy to do in live fish. "Robotic devices also facilitate three-dimensional kinematic studies and correlated hydrodynamic analyses, as the location of the locomotor surface can be known accurately. And, individual components of a natural motion (such as outstroke vs. instroke of a flapping appendage) can be programmed separately, which is certainly difficult to achieve when working with a live animal."[82]
Diversity of fins
See also
- Cephalopod fin
- Fin and flipper locomotion
- Fish locomotion
- Polydactyly in early tetrapods
- RoboTuna
- Shark fin soup
- Shark finning
- Tradeoffs for locomotion in air and water
- Undulatory locomotion
References
Citations
- ↑ Standen EM (2009) "Muscle activity and hydrodynamic function of pelvic fins in trout (Oncorhynchus mykiss) The Journal of Experimental Biology, 213: 831–841. doi:10.1242/jeb.033084
- ↑ Tytell, E. (2005). "The Mysterious Little Fatty Fin". Journal of Experimental Biology 208 (1): v. doi:10.1242/jeb.01391.
- ↑ Reimchen, T E; Temple, N F (2004). "Hydrodynamic and phylogenetic aspects of the adipose fin in fishes". Canadian Journal of Zoology 82 (6): 910–916. doi:10.1139/Z04-069.
- ↑ Temple, Nicola (18 July 2011). "Removal of trout, salmon fin touches a nerve". Cosmos.
- ↑ Buckland-Nicks, J. A.; Gillis, M.; Reimchen, T. E. (2011). "Neural network detected in a presumed vestigial trait: ultrastructure of the salmonid adipose fin". Proceedings of the Royal Society B: Biological Sciences 279 (1728): 553–563. doi:10.1098/rspb.2011.1009.
- ↑ Stewart, Thomas A.; Smith, W. Leo; Coates, Michael I. (2014). "The origins of adipose fins: an analysis of homoplasy and the serial homology of vertebrate appendages". Proceedings of the Royal Society B: Biological Sciences 281 (1781): 20133120. doi:10.1098/rspb.2013.3120.
- ↑ von Zittel KA, Woodward AS and Schlosser M (1932) Text-book of Paleontology Volume 2, Macmillan and Company. Page 13.
- ↑ Clack, J. A. (2002) Gaining Ground. Indiana University
- ↑ Johanson, Zerina, John A. Long, John A. Talent, Philippe Janvier, and James W. Warren (2006) "Oldest Coelacanth, from the Early Devonian of Australia" Biology Letters, 2 (3): 443–46.
- ↑ Forey 1998.
- ↑ Fricke, Hans, Olaf Reinicke, Heribert Hofer, and Werner Nachtigall. "Locomotion of the Coelacanth Latimeria Chalumnae in Its Natural Environment." Nature 329.6137 (1987): 331–33. Print.
- ↑ Durán, I.; Marí-Beffa, M.; Santamaría, J. A.; Becerra, J.; Santos-Ruiz, L. (2011). "Actinotrichia collagens and their role in fin formation". Developmental Biology 354 (1): 160–172. doi:10.1016/j.ydbio.2011.03.014. PMID 21420398.
- ↑ Zhang, J.; Wagh, P.; Guay, D.; Sanchez-Pulido, L.; Padhi, B. K.; Korzh, V.; Andrade-Navarro, M. A.; Akimenko, M. A. (2010). "Loss of fish actinotrichia proteins and the fin-to-limb transition". Nature 466 (7303): 234–237. Bibcode:2010Natur.466..234Z. doi:10.1038/nature09137. PMID 20574421.
- ↑ Hamlett 1999, p. 528.
- ↑ Function of the heterocercal tail in sharks: quantitative wake dynamics during steady horizontal swimming and vertical maneuvering - The Journal of Experimental Biology 205, 2365–2374 (2002)
- ↑ "A Shark's Skeleton & Organs". Archived from the original on April 9, 2012. Retrieved August 14, 2009.
- ↑ Michael, Bright. "Jaws: The Natural History of Sharks". Columbia University. Retrieved 2009-08-29.
- ↑ Nelson, Joseph S. (1994). Fishes of the World. New York: John Wiley and Sons. ISBN 0-471-54713-1. OCLC 28965588.
- ↑ 19.0 19.1 19.2 Sfakiotakis M, Lane DM and Davies JBC (1999) "Review of Fish Swimming Modes for Aquatic Locomotion" IEEE Journal of Oceanic Engineering, 24 (2).
- ↑ Franc, Jean-Pierre and Michel, Jean-Marie (2004) Fundamentals of Cavitation Springer. ISBN 9781402022326.
- ↑ 21.0 21.1 Brahic, Catherine (2008-03-28). "Dolphins swim so fast it hurts". NewScientist. Retrieved 2008-03-31.
- ↑ Nauen JC, Lauder GV (2001a) "Locomotion in scombrid fishes: visualization of flow around the caudal peduncle and finlets of the Chub mackerel Scomber japonicus" Journal of Experimental Biology, 204: 2251–63.
- ↑ Nauen JC, Lauder GV (2001b) "Three-dimensional analysis of finlet kinematics in the Chub mackerel (Scomber japonicus)" The Biological Bulletin, 200: 9–19.
- ↑ Nauen JC and Lauder GV (2000) "Locomotion in scombrid fishes: morphology and kinematics of the finlets of the Chub mackerel Scomber japonicus" Journal of Experimental Biology, 203: 2247–59.
- ↑ Flammang BE, Lauder GV, Troolin DR and Strand TE (2011) "Volumetric imaging of fish locomotion" Biology Letters, 7: 695–698. doi:10.1098/rsbl.2011.0282
- ↑ Fish FE and Lauder GV (2006) "Passive and active flow control by swimming fishes and mammals" Annual Review of Fluid Mechanics, 38: 193–224. doi:10.1146/annurev.fluid.38.050304.092201
- ↑ Magnuson JJ (1978) "Locomotion by scombrid fishes: Hydromechanics, morphology and behavior" in Fish Physiology, Volume 7: Locomotion, WS Hoar and DJ Randall (Eds) Academic Press. Page 240–308. ISBN 9780123504074.
- ↑ Ship's movements at sea Retrieved 22 November 2012.
- ↑ Rana and Joag (2001) Classical Mechanics Page 391, Tata McGraw-Hill Education. ISBN 9780074603154.
- ↑ 30.0 30.1 30.2 Alevizon WS (1994) "Pisces Guide to Caribbean Reef Ecology" Gulf Publishing Company ISBN 1-55992-077-7
- ↑ Lingham-Soliar, T. (2005). "Dorsal fin in the white shark,Carcharodon carcharias: A dynamic stabilizer for fast swimming". Journal of Morphology 263 (1): 1–11. doi:10.1002/jmor.10207. PMID 15536651.
- ↑ 32.0 32.1 Ichthyology Florida Museum of Natural History. Retrieved 22 November 2012.
- ↑ Masterson, J. "Gambusia affinis". Smithsonian Institution. Retrieved 21 October 2011.
- ↑ Kuntz, Albert (1913). "Notes on the Habits, Morphology of the Reproductive Organs, and Embryology of the Viviparous Fish Gambusia affinis". Bulletin of the United States Bureau of Fisheries (Department of Commerce) 33: 181–190.
- ↑ Kapoor BG and Khanna B (2004) Ichthyology Handbook pp. 497–498, Springer Science & Business Media. ISBN 9783540428541.
- ↑ Helfman G, Collette BB, Facey DH and Bowen BW (2009) The Diversity of Fishes: Biology, Evolution, and Ecology p. 35, Wiley-Blackwell. ISBN 978-1-4051-2494-2.
- ↑ "System glossary". FishBase. Retrieved 2013-02-15.
- ↑ Heinicke, Matthew P.; Naylor, Gavin J. P.; Hedges, S. Blair (2009). The Timetree of Life: Cartilaginous Fishes (Chondrichthyes). Oxford University Press. p. 320. ISBN 0191560154.
- ↑ 39.0 39.1 Aquatic Life of the World pp. 332–333, Marshall Cavendish Corporation, 2000. ISBN 9780761471707.
- ↑ Dement J Species Spotlight: Atlantic Sailfish (Istiophorus albicans) littoralsociety.org. Retrieved 1 April 2012.
- ↑ Bertelsen E and Pietsch TW (1998). Encyclopedia of Fishes. San Diego: Academic Press. pp. 138–139. ISBN 0-12-547665-5.
- ↑ Purple Flying Gurnard, Dactyloptena orientalis (Cuvier, 1829) Australian Museum. Updated: 15 September 2012. Retrieved: 2 November 2012.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2012). "Dactyloptena orientalis" in FishBase. November 2012 version.
- ↑ Female fish flaunt fins to attract a mate ScienceDaily. 8 October 2010.
- ↑ Baldauf SA, TCM Bakker, F Herder, H Kullmann and T Thünken (2010) "Male mate choice scales female ornament allometry in a cichlid fish" BMC Evolutionary Biologr//, 10 :301. doi:10.1186/1471-2148-10-301
- ↑ Schultz, Ken (2011) Ken Schultz's Field Guide to Saltwater Fish Page 250, John Wiley & Sons. ISBN 9781118039885.
- ↑ Vannuccini S (1999). "Shark utilization, marketing and trade". FAO Fisheries Technical Paper (Rome: FAO) 389.
- ↑ Moore, John A (1988) [www.sicb.org/dl/saawok/449.pdf "Understanding nature—form and function"] Page 485, American Zoologist, 28: 449–584.
- ↑ Evolution Of Fins And Limbs Linked With That Of Gills ScienceDaily. 25 March 2009.
- ↑ Gillis JA, RD Dahn and NH Shubin (2009) "Shared developmental mechanisms pattern the vertebrate gill arch and paired fin skeletons" Proceedings of the National Academy of Sciences, 106 (14): 5720–5724.
- ↑ Wings, legs, and fins: How do new organs arise in evolution? Neil Shubin, University of Chicago.
- ↑ "Primordial Fish Had Rudimentary Fingers" ScienceDaily, 23 September 2008.
- ↑ Hall, Brian K (2007) Fins into Limbs: Evolution, Development, and Transformation University of Chicago Press. ISBN 9780226313375.
- ↑ Shubin, Neil (2009) Your inner fish: A journey into the 3.5 billion year history of the human body Vintage Books. ISBN 9780307277459. UCTV interview
- ↑ Clack, Jennifer A (2012) "From fins to feet" Chapter 6, pages 187–260, in: Gaining Ground, Second Edition: The Origin and Evolution of Tetrapods, Indiana University Press. ISBN 9780253356758.
- ↑ Lungfish Provides Insight to Life On Land: 'Humans Are Just Modified Fish' ScienceDaily, 7 October 2011.
- ↑ Cole NJ, Hall TE, Don EK, Berger S, Boisvert CA, et al. (2011) "Development and Evolution of the Muscles of the Pelvic Fin" PLoS Biology, 9 (10): e1001168. doi:10.1371/journal.pbio.1001168

- ↑ A small step for lungfish, a big step for the evolution of walking" ScienceDaily, 13 December 2011.
- ↑ King HM, NH Shubin, MI Coates and Hale ME (2011) "Behavioral evidence for the evolution of walking and bounding before terrestriality in sarcopterygian fishes" Proceedings of the National Academy of Sciences, 108 (52): 21146–21151.
- ↑ Shubin N, C Tabin, S Carroll (1997) "Fossils, genes and the evolution of animal limbs" Review article, Nature, 388: 639–648.
- ↑ Vertebrate flight: The three solutions University of California. Updated 29 September 2005.
- ↑ "Scientists find missing link between the dolphin, whale and its closest relative, the hippo". Science News Daily. 2005-01-25. Retrieved 2007-06-18.
- ↑ Gatesy, J. (1 May 1997). "More DNA support for a Cetacea/Hippopotamidae clade: the blood-clotting protein gene gamma-fibrinogen". Molecular Biology and Evolution 14 (5): 537–543. doi:10.1093/oxfordjournals.molbev.a025790. PMID 9159931.
- ↑ John J. Flynn et al. (2005). "Molecular Phylogeny of the Carnivora". Systematic Biology 54 (2): 317–337. doi:10.1080/10635150590923326. PMID 16012099. Missing
|last3=in Authors list (help) - ↑ Felts WJL "Some functional and structural characteristics of cetacean flippers and flukes" Pages 255–275 in: Norris KS (ed.) Whales, Dolphins, and Porpoises, University of California Press.
- ↑ The evolution of whales University of California Museum. Retrieved 27 November 2012.
- ↑ Thewissen JGM, Cooper LN, George JC and Bajpai S (2009) "From Land to Water: the Origin of Whales, Dolphins, and Porpoises" Evo Edu Outreach, 2: 272–288. doi:10.1007/s12052-009-0135-2
- ↑ Martill D.M. (1993). "Soupy Substrates: A Medium for the Exceptional Preservation of Ichthyosaurs of the Posidonia Shale (Lower Jurassic) of Germany". Kaupia - Darmstädter Beiträge zur Naturgeschichte, 2 : 77-97.
- ↑ Gould,Stephen Jay (1993 "Bent Out of Shape" in Eight Little Piggies: Reflections in Natural History. Norton, 179–94. ISBN 9780393311396.
- ↑ Richard Mason. "What is the market for robot fish?".
- ↑ Witoon Juwarahawong. "Fish Robot". Institute of Field Robotics. Archived from the original on 2007-11-04. Retrieved 2007-10-25.
- ↑ "Robotic fish powered by Gumstix PC and PIC". Human Centred Robotics Group at Essex University. Retrieved 2007-10-25.
- ↑ "Robotic fish make aquarium debut". cnn.com. CNN. 10 October 2005. Retrieved 12 June 2011.
- ↑ Walsh, Dominic (3 May 2008). "Merlin Entertainments tops up list of London attractions with aquarium buy". thetimes.co.uk. Times of London. Retrieved 12 June 2011.
- ↑ For Festo, Nature Shows the Way Control Engineering, 18 May 2009.
- ↑ Bionic penguins fly through water... and air Gizmag, 27 April 2009.
- ↑ Festo AquaRay Robot Technovelgy, 20 April 2009.
- ↑ The AquaJelly Robotic Jellyfish from Festo Engineering TV, 12 July 2012.
- ↑ Lightweight robots: Festo's flying circus The Engineer, 18 July 2011.
- ↑ Huge Herr, D. Robert G "A Swimming Robot Actuated by Living Muscle Tissue" Journal of NeuroEngineering and Rehabilitation, 1: 6. doi:10.1186/1743-0003-1-6
- ↑ How Biomechatronics Works HowStuffWorks/ Retrieved 22 November 2012.
- ↑ Lauder, G. V. (2011) "Swimming hydrodynamics: ten questions and the technical approaches needed to resolve them" Experiments in fluids, 51 (1): 23–35.
Bibliography
- Hamlett, William C. (1999). Sharks, skates, and rays: the biology of elasmobranch fishes (1 ed.). p 56: The Johns Hopkins University Press. ISBN 978-0-8018-6048-5.
Further reading
- Hall, Brian K (2007) Fins into Limbs: Evolution, Development, and Transformation University of Chicago Press. ISBN 9780226313375.
- Helfman G, Collette BB, Facey DE and Bowen BW (2009) "Functional morphology of locomotion and feeding" Chapter 8, pp. 101–116. In:The Diversity of Fishes: Biology, John Wiley & Sons. ISBN 9781444311907.
- Lauder GV, Nauen JC and Drucker EG (2002) "Experimental Hydrodynamics and Evolution: Function of Median Fins in Ray-finned Fishes" Integr. Comp. Biol. 42 (5): 1009–1017. doi:10.1093/icb/42.5.1009
- Lauder GV, EG Drucker (2004) "Morphology and experimental hydrodynamics of fish fin control surfaces" Journal of Oceanic Engineering, 29 (3): 556–571.
External links
- Fish anatomy Mongabay.
- Homology of fin lepidotrichia in osteichthyan fishes
- Generalized fish tail and fin types University of California.
- The Fish's Fin Earthlife Web
- Can robot fish find pollution? HowStuffWorks. Accessed 30 January 2012.
| ||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||