Fibrillarin
| Fibrillarin | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 2ipx. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | FBL ; FIB; FLRN; RNU3IP1 | ||||||||||||
| External IDs | OMIM: 134795 MGI: 95486 HomoloGene: 1099 GeneCards: FBL Gene | ||||||||||||
| |||||||||||||
| RNA expression pattern | |||||||||||||
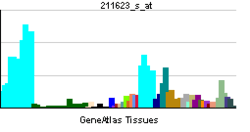 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | 2091 | 14113 | |||||||||||
| Ensembl | ENSG00000105202 | ENSMUSG00000046865 | |||||||||||
| UniProt | P22087 | P35550 | |||||||||||
| RefSeq (mRNA) | NM_001436 | NM_007991 | |||||||||||
| RefSeq (protein) | NP_001427 | NP_032017 | |||||||||||
| Location (UCSC) | Chr 19: 40.33 – 40.34 Mb | Chr 7: 28.17 – 28.18 Mb | |||||||||||
| PubMed search | |||||||||||||
| Fibrillarin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 pyrococcus horikoshii fibrillarin pre-rrna processing protein | |||||||||
| Identifiers | |||||||||
| Symbol | Fibrillarin | ||||||||
| Pfam | PF01269 | ||||||||
| Pfam clan | CL0063 | ||||||||
| InterPro | IPR000692 | ||||||||
| PROSITE | PDOC00489 | ||||||||
| SCOP | 1fbn | ||||||||
| SUPERFAMILY | 1fbn | ||||||||
| |||||||||
rRNA 2'-O-methyltransferase fibrillarin is an enzyme that in humans is encoded by the FBL gene.[1][2][3]
This gene product is a component of a nucleolar small nuclear ribonucleoprotein (snRNP) particle thought to participate in the first step in processing pre-ribosomal (r)RNA. It is associated with the U3, U8, and U13 small nucleolar RNAs and is located in the dense fibrillar component (DFC) of the nucleolus. The encoded protein contains an N-terminal repetitive domain that is rich in glycine and arginine residues, like fibrillarins in other species. Its central region resembles an RNA-binding domain and contains an RNP consensus sequence. Antisera from approximately 8% of humans with the autoimmune disease scleroderma recognize fibrillarin.[3]
Fibrillarin is a component of several ribonucleoproteins including a nucleolar small nuclear ribonucleoprotein (SnRNP) and one of the two classes of small nucleolar ribonucleoproteins (snoRNPs). SnRNAs function in RNA splicing while snoRNPs function in ribosomal RNA processing.
Fibrillarin is associated with U3, U8 and U13 small nuclear RNAs in mammals and is similar to the yeast NOP1 protein. Fibrillarin has a well conserved sequence of around 320 amino acids, and contains 3 domains, an N-terminal Gly/Arg-rich region; a central domain resembling other RNA-binding proteins and containing an RNP-2-like consensus sequence; and a C-terminal alpha-helical domain. An evolutionarily related pre-rRNA processing protein, which lacks the Gly/Arg-rich domain, has been found in various archaebacteria.
A study by Schultz et al. indicated that the K-turn binding 15.5-kDa protein (called Snu13 in yeast) interacts with spliceosome proteins hPRP31, hPRP3, hPRP4, CYPH and the small nucleolar ribonucleoproteins NOP56, NOP58, and fibrillarin. The 15.5-kDa protein has sequence similarity to other RNA-binding proteins such as ribosomal proteins S12, L7a, and L30 and the snoRNP protein NHP2. The U4/U6 snRNP contains 15.5-kDa protein.[4] The 15.5-kDa protein also exists in a ribonucleoprotein complex that binds the U3 box B/C motif. The 15.5-kDa protein also exists as one of the four core proteins of the C/D small nucleolar ribonucleoprotein that mediates methylation of pre-ribosomal RNAs.
Structural evidence supporting the idea that fibrillarin is the snoRNA methyltransferase has been reviewed.[5]
Interactions
Fibrillarin has been shown to interact with DDX5[6] and SMN1.[7]
References
- ↑ Aris JP, Blobel G (March 1991). "cDNA cloning and sequencing of human fibrillarin, a conserved nucleolar protein recognized by autoimmune antisera". Proc. Natl. Acad. Sci. U.S.A. 88 (3): 931–5. doi:10.1073/pnas.88.3.931. PMC 50928. PMID 1846968.
- ↑ Jansen RP, Hurt EC, Kern H, Lehtonen H, Carmo-Fonseca M, Lapeyre B, Tollervey D (June 1991). "Evolutionary conservation of the human nucleolar protein fibrillarin and its functional expression in yeast". J Cell Biol 113 (4): 715–29. doi:10.1083/jcb.113.4.715. PMC 2288999. PMID 2026646.
- ↑ 3.0 3.1 "Entrez Gene: FBL fibrillarin".
- ↑ Protein-Protein and Protein-RNA Contacts both Contribute to the 15.5K-Mediated Assembly of the U4/U6 snRNP and the Box C/D snoRNPs by Annemarie Schultz, Stephanie Nottrott, Nicholas James Watkins and Reinhard Lührmann in Molecular and Cellular Biology (2006) Volume 26, pages 5146–5154.
- ↑ The structure and function of small nucleolar ribonucleoproteins by Steve L. Reichow, Tomoko Hamma, Adrian R. Ferré-D'Amaré and Gabriele Varani in Nucleic Acids Research (2007) Volume 35, pages 1452–1464.
- ↑ Nicol, S M; Causevic M; Prescott A R; Fuller-Pace F V (June 2000). "The nuclear DEAD box RNA helicase p68 interacts with the nucleolar protein fibrillarin and colocalizes specifically in nascent nucleoli during telophase". Exp. Cell Res. (UNITED STATES) 257 (2): 272–80. doi:10.1006/excr.2000.4886. ISSN 0014-4827. PMID 10837141.
- ↑ Pellizzoni, L; Baccon J; Charroux B; Dreyfuss G (July 2001). "The survival of motor neurons (SMN) protein interacts with the snoRNP proteins fibrillarin and GAR1". Curr. Biol. (England) 11 (14): 1079–88. doi:10.1016/S0960-9822(01)00316-5. ISSN 0960-9822. PMID 11509230.
Further reading
- Baserga SJ, Yang XD, Steitz JA (1991). "An intact Box C sequence in the U3 snRNA is required for binding of fibrillarin, the protein common to the major family of nucleolar snRNPs". EMBO J. 10 (9): 2645–51. PMC 452965. PMID 1714385.
- Okano Y, Steen VD, Medsger TA (1992). "Autoantibody to U3 nucleolar ribonucleoprotein (fibrillarin) in patients with systemic sclerosis". Arthritis Rheum. 35 (1): 95–100. doi:10.1002/art.1780350114. PMID 1731817.
- Lischwe MA; Ochs RL; Reddy R et al. (1985). "Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG,NG-dimethylarginine". J. Biol. Chem. 260 (26): 14304–10. PMID 2414294.
- Méhes G, Pajor L (1995). "Nucleolin and fibrillarin expression in stimulated lymphocytes and differentiating HL-60 cells. A flow cytometric assay". Cell Prolif. 28 (6): 329–36. doi:10.1111/j.1365-2184.1995.tb00074.x. PMID 7626687.
- Liu Q, Dreyfuss G (1996). "A novel nuclear structure containing the survival of motor neurons protein". EMBO J. 15 (14): 3555–65. PMC 451956. PMID 8670859.
- Magoulas C; Zatsepina OV; Jordan PW et al. (1998). "The SURF-6 protein is a component of the nucleolar matrix and has a high binding capacity for nucleic acids in vitro". Eur. J. Cell Biol. 75 (2): 174–83. doi:10.1016/s0171-9335(98)80059-9. PMID 9548374.
- Ai LS, Lin CH, Hsieh M, Li C (1999). "Arginine methylation of a glycine and arginine rich peptide derived from sequences of human FMRP and fibrillarin". Proc. Natl. Sci. Counc. Repub. China B 23 (4): 175–80. PMID 10518318.
- Pintard L, Kressler D, Lapeyre B (2000). "Spb1p Is a Yeast Nucleolar Protein Associated with Nop1p and Nop58p That Is Able To Bind S-Adenosyl-l-Methionine In Vitro". Mol. Cell. Biol. 20 (4): 1370–81. doi:10.1128/MCB.20.4.1370-1381.2000. PMC 85287. PMID 10648622.
- Nicol SM, Causevic M, Prescott AR, Fuller-Pace FV (2000). "The nuclear DEAD box RNA helicase p68 interacts with the nucleolar protein fibrillarin and colocalizes specifically in nascent nucleoli during telophase". Exp. Cell Res. 257 (2): 272–80. doi:10.1006/excr.2000.4886. PMID 10837141.
- Pellizzoni L, Baccon J, Charroux B, Dreyfuss G (2001). "The survival of motor neurons (SMN) protein interacts with the snoRNP proteins fibrillarin and GAR1". Curr. Biol. 11 (14): 1079–88. doi:10.1016/S0960-9822(01)00316-5. PMID 11509230.
- Zhou X; Tan FK; Xiong M et al. (2001). "Systemic sclerosis (scleroderma): specific autoantigen genes are selectively overexpressed in scleroderma fibroblasts". Journal of Immunology 167 (12): 7126–33. doi:10.4049/jimmunol.167.12.7126. PMID 11739535.
- Andersen JS; Lyon CE; Fox AH et al. (2002). "Directed proteomic analysis of the human nucleolus". Curr. Biol. 12 (1): 1–11. doi:10.1016/S0960-9822(01)00650-9. PMID 11790298.
- Cimato TR; Tang J; Xu Y et al. (2002). "Nerve growth factor-mediated increases in protein methylation occur predominantly at type I arginine methylation sites and involve protein arginine methyltransferase 1". J. Neurosci. Res. 67 (4): 435–42. doi:10.1002/jnr.10123. PMID 11835310.
- Fujiyama S; Yanagida M; Hayano T et al. (2002). "Isolation and proteomic characterization of human Parvulin-associating preribosomal ribonucleoprotein complexes". J. Biol. Chem. 277 (26): 23773–80. doi:10.1074/jbc.M201181200. PMID 11960984.
- Whitehead SE; Jones KW; Zhang X et al. (2003). "Determinants of the interaction of the spinal muscular atrophy disease protein SMN with the dimethylarginine-modified box H/ACA small nucleolar ribonucleoprotein GAR1". J. Biol. Chem. 277 (50): 48087–93. doi:10.1074/jbc.M204551200. PMID 12244096.
- Herrera-Esparza R; Kruse L; von Essen M et al. (2003). "U3 snoRNP associates with fibrillarin a component of the scleroderma clumpy nucleolar domain". Arch. Dermatol. Res. 294 (7): 310–7. doi:10.1007/s00403-002-0338-7. PMID 12373336.
- Chen M; Rockel T; Steinweger G et al. (2003). "Subcellular Recruitment of Fibrillarin to Nucleoplasmic Proteasomes: Implications for Processing of a Nucleolar Autoantigen". Mol. Biol. Cell 13 (10): 3576–87. doi:10.1091/mbc.02-05-0083. PMC 129967. PMID 12388758.
- Watkins NJ, Dickmanns A, Lührmann R (2003). "Conserved Stem II of the Box C/D Motif Is Essential for Nucleolar Localization and Is Required, Along with the 15.5K Protein, for the Hierarchical Assembly of the Box C/D snoRNP". Mol. Cell. Biol. 22 (23): 8342–52. doi:10.1128/MCB.22.23.8342-8352.2002. PMC 134055. PMID 12417735.
| |||||||||