Fasciola hepatica
| Fasciola hepatica | |
|---|---|
| | |
| Fasciola hepatica – adult worm | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Platyhelminthes |
| Class: | Trematoda |
| Subclass: | Digenea |
| Order: | Echinostomida |
| Suborder: | Distomata |
| Family: | Fasciolidae |
| Genus: | Fasciola |
| Species: | F. hepatica |
| Binomial name | |
| Fasciola hepatica Linnaeus, 1758 | |
Fasciola hepatica, also known as the common liver fluke or sheep liver fluke, is a parasitic trematod ( fluke or flatworm, a type of helminth) of the class Trematoda, phylum Platyhelminthes that infects the livers of various mammals, including humans. The disease caused by the fluke is called fascioliasis (also known as fasciolosis), which is a type of helminthiasis and has been classified as a neglected tropical disease.[1] F. hepatica is distributed worldwide, and causes great economic losses in sheep and cattle. It has been known as an important parasite of sheep and cattle for hundreds of years. Because of its size and economic importance, it has been the subject of many scientific investigations and may be the best-known of any trematode species.
Morphology
Fasciola hepatica is one of the largest flukes of the world, reaching a length of 30 mm and a width of 13 mm. It is leaf-shape, pointed at the end or posteriorly, and wide in the front or anteriorly, although the shape varies somewhat. The oral sucker is small but powerful and is located at the end of a cone-shape projection at the anterior end. The acetabulum is larger than the oral sucker and is anterior. The internal tegument is covered with large, and scalelike spines. The intestinal ceca are highly dendritic and extend to near the posterior end of the body. The testes are large and greatly branched, arranged in tandem behind the ovary. The smaller, dendritic ovary lies on the right side, coiling between the ovary and the preacetabular cirrus pouch. Vitelline follicles are extensive, filling most of the lateral body and becoming confluent behind the testes.
Life cycle
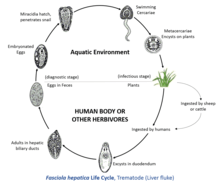
To complete its life cycle, F. hepatica requires a freshwater snail as an intermediate host, such as Galba truncatula, in which the parasite can reproduce asexually.
Species in the family of air-breathing freshwater snails, the Lymnaeidae that serve as naturally or experimentally intermediate hosts of Fasciola hepatica include: Austropeplea tomentosa, Austropeplea ollula, Austropeplea viridis, Radix peregra, Radix lagotis, Radix auricularia, Radix natalensis, Radix rubiginosa, Omphiscola glabra, Lymnaea stagnalis, Stagnicola fuscus, Stagnicola palustris, Stagnicola turricula, Pseudosuccinea columella, Lymnaea viatrix, Lymnaea neotropica, Fossaria bulimoides, Lymnaea cubensis, Lymnaea sp. from Colombia, Galba truncatula, Lymnaea cousini, Lymnaea humilis, Lymnaea diaphana, Stagnicola caperata, and Lymnaea occulta.[2]
The metacercariae are ingested by the ruminant or, in some cases, by humans eating uncooked foods such as watercress or salad. Contact with low pH in the stomach causes the early immature juvenile to excyst. In the duodenum, the parasite breaks free of the metacercariae and burrows through the intestinal lining into the peritoneal cavity. The newly excysted juvenile does not feed at this stage, but, once it finds the liver parenchyma after a period of days, feeding will start. This immature stage in the liver tissue is the pathogenic stage, causing anaemia and clinical signs sometimes observed in infected animals or humans. The parasite browses on liver tissu, on the lining of biliary ducts, for a period of up to six weeks, and eventually finds its way to the large bile duct, where it matures into an adult. Adult hepatica lives in small passages of the liver of many kinds of mammals, especially ruminants and begins to produce eggs. Up to 25,000 eggs per day per fluke can be produced, and, in a light infection, up to 500,000 eggs per day can be deposited onto pasture by a single sheep.
Eggs are passed out of the liver with bile and into the intestine to be voided with feces. If they fall into water, eggs will complete their development into miracidia and hatch in 9 to 10 days during warm weather. Colder water retards their development. On hatching, miracidia have 24 hours in which they have to find a suitable snail host. Mother sporocysts produce first-generation rediae, which in turn produce daughter rediae that develop in the snail's digestive gland. From the snail, minute cercariae emerge and swim through pools of water in pasture, and encyst as metacercariae on near-by vegetation.
Epidemiology
Infection begins when metacercaria - infected aquatic vegetation is eaten or when water containing metacercariae is drunk. In the United Kingdom, fasciola frequently causes disease in ruminants, most commonly between March and December.[3]
Humans are often infected by eating watercress or drinking 'Emoliente', a Peruvian drink that uses drops of watercress juice.[3] Cattle and sheep are infected when they consume the infectious stage of the parasite from low-lying, marshy pasture.
Human infections occur in parts of Europe, like England and Ireland, Northern Iran, northern Africa, like Egypt, in Cuba, South America, especially the Altiplano regions of the Peruvian and Bolivian Andes and are emerging in Vietnam and Cambodia. As of 2014, the prevalence in children between 3 and 12 years was 11% by stool microscopy and ELISA in the cattle farming areas near Cusco, Peru. Risk factors were number of siblings in the household, drinking untreated water, and giardiasis.[4]
It is one of the most important disease agents of domestic stock throughout the world and likely will remain so in the future.
Pathology
Little damage is done by juveniles penetrating the intestinal wall and the capsule surrounding the liver but much necrosis results from migration of flukes through the liver parenchyma. During this time, they feed on liver cells and blood. Anemia sometimes results from heavy infections. Worms in bile ducts cause inflammation and edema, which in turn stimulate production of fibrous tissue in the walls of these ducts. Thus thickened, the ducts can handle less bile and are less responsive to needs of the liver. Back pressure causes atrophy of liver parenchyma, with concomitant cirrhosis and possibly jaundice. In heavy infections, the gallbladder is damaged, and walls of the bile ducts are eroded completely. Fascioliasis is one of the major causes of hypereosinophilia in France.
Clinical manifestations

The effects of liver flukes are referred to as fascioliasis, and include anaemia, weight loss, and submandibular oedema; diarrhoea is only an occasional consequence.
A serious consequence of the liver damage caused by fascioliasis is that latent Clostridium novyi spores can be activated by the low-oxygen conditions in the damaged tracts the parasite forms in the liver; this can lead to "black disease", caused by Clostridium novyi type B or immune-mediated haemolytic anaemia (IMHA) leading to haemoglobinuria caused by C. novyi type D.
One distinguishes between an acute and chronic phases. The acute phase of migrating parenchymal larvae causes fever, eosinophilia, right upper quadrant pain and especially loss of apetite. Vomiting and weight loss of 20 kg or more may develop, and abates when the larvae mature to adults. The adult flukes in the biliary tree are generally asymptomatic. Some patients develop chronic disease with right upper quadrant pain, nausea, vomiting, and hepatomegaly. Eosinophilia and abnormal liver function are less common than in acute disease. Anatomically adult flukes can cause hyperplasia, desquamation, thickening, and widening of the bile ducts. No malignant degeneration and cholangiocarcinoma has been observed. a case series with clinical findings and evolution of disease[5]
Diagnosis

A diagnosis may be made by finding yellow-brown eggs in the stool. They are indistinguishable from the eggs of Fascioloides magna, although the eggs of F. magna are very rarely passed in sheep, goats, or cattle. A false record can result when the patient has eaten infected liver and egg passes through the feces. Daily examination during a liver-free diet will unmask the false diagnosis.
An enzyme-linked immunosorbent assay (ELISA) test is the test of choice. The ELISA is available commercially and can detect anti-hepatica antibodies in serum and milk; new ones especially intended for use on fecal samples are being developed. ELISA is more specific than Western blot or Arc2 immunodiffusion.[3]Proteases secreted by F. hepatica have been used experimentally in immunizing antigens.
Treatment
Several drugs are effective in chemotherapy of fascioliasis, both in humans and in domestic animals. The drug of choice in the treatment of fasciolosis is triclabendazole, a member of the benzimidazole family of anthelmintics. The drug works by preventing the polymerization of the molecule tubulin into the cytoskeletal structures, microtubules. Resistance of F. hepatica to triclabendazole has been recorded in Australia in 1995[6] and Ireland in 1998.[7] triclabendazole is on the WHO's essential drugs list and is available via free donation from the WHO. In the U.S. the CDC Parasitic Drug Service dispenses the drug.[3] Rafoxanide acts by uncoupling oxidative phosphorylation in the fluke. Artemether has been shown to be effective in a rat model of fascioliasis.[8]
See also
- List of parasites (human)
References
- ↑ "Neglected Tropical Diseases". cdc.gov. June 6, 2011. Retrieved 28 November 2014.
- ↑ Correa C. A., Escobar J. S., Durand P., Renaud F., David P., Jarne P., Pointier J.-P. & Hurtrez-Boussès S. (2010). "Bridging gaps in the molecular phylogeny of the Lymnaeidae (Gastropoda: Pulmonata), vectors of Fascioliasis". BMC Evolutionary Biology 10: 381. doi:10.1186/1471-2148-10-381.
- ↑ 3.0 3.1 3.2 3.3 "Gorgas Case 5 - 2015 Series". The Gorgas Course in Clinical Tropical Medicine. University of Alabama. 2 March 2015. Retrieved 10 March 2015.
- ↑ Cabada MM, Goodrich MR, Graham B, Villanueva-Meyer PG, Lopez 2, Arque E, White AC Jr. Fascioliasis and eosinophilia in the highlands of Cuzco, Peru and their association with water and socioeconomic factors. Am J Trop Med Hyg. 2014 Nov;91(5):989-93. PMID 25200257. doi: 10.4269/ajtmh.14-0169.
- ↑ Marcos LA, Tagle M, Terashima A, Bussalleu A, Ramirez C, Carrasco C, Valdez L, Huerta-Mercado J, Freedman DO, Vinetz JM, Gotuzzo E. [Natural history, clinicoradiologic correlates, and response to triclabendazole in acute massive fascioliasis.http://www.ncbi.nlm.nih.gov/pubmed/18256419] Am J Trop Med Hyg. 2008 Feb;78(2):222-7.PMID 18256419
- ↑ Overend DJ & Bowen FL (1995). "Resistance of Fasciola hepatica to triclabendazole". Austral Vet J 72 (7): 275–276. doi:10.1111/j.1751-0813.1995.tb03546.x. PMID 8534235.
- ↑ Mulcahy G & Dalton JP (1998). "Vaccines in control of liver fluke infections in ruminants: current status and prospects". Irish Vet J 51: 520–525.
- ↑ Keiser J, Utzinger J, Vennerstrom JL et al. (2007). "Activity of artemether and OZ78 against triclabendazole-resistant Fasciola hepatica". Trans R Soc Trop Med Hyg 101 (12): 1219–1222. doi:10.1016/j.trstmh.2007.07.012. PMID 17905370.
External links
- Animal Diversity Web University of Michigan
- Parasites in humans
- Parasite Parasite .org Australia
- Stanford University
- Taxonomic classification
- Encyclopedia of Life
- Taxonomy and nomenclature at ITIS.gov
- Molecular database at UniProt
| Wikimedia Commons has media related to Fasciola hepatica. |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||