Farnesol
 | |
 | |
| Names | |
|---|---|
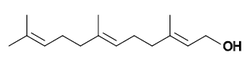
| IUPAC name
(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol | |
| Identifiers | |
| 4602-84-0 | |
| ChEBI | CHEBI:28600 |
| ChEMBL | ChEMBL25308 |
| ChemSpider | 3210 392816 (2E,6E)- |
| DrugBank | DB02509 |
| |
| Jmol-3D images | Image Image (2E,6E)- |
| KEGG | C01493 |
| PubChem | 3327 445070 (2E,6E)- |
| |
| UNII | X23PI60R17 |
| Properties | |
| Molecular formula |
C15H26O |
| Molar mass | 222.37 g·mol−1 |
| Density | 0.887 g/cm3 |
| Boiling point | 283 to 284.00 °C (541.40 to 543.20 °F; 556.15 to 557.15 K) at 760 mmHg 111 °C at 0.35 mmHg |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Farnesol is a natural 15-carbon organic compound which is an acyclic sesquiterpene alcohol. Under standard conditions it is a colorless liquid. It is hydrophobic, and thus insoluble in water, but miscible with oils.
Farnesol is produced from 5-carbon isoprene compounds in both plants and animals. Phosphate activated derivatives of farnesol are the building blocks of most, and possibly all, acyclic sesquiterpenoids. These compounds are doubled to form 30-carbon squalene, which in turn is the precursors for steroids in plants, animals, and fungi. As such, farnesol and its derivatives are important starting compounds for both natural and artificial organic synthesis.
Uses
Farnesol is present in many essential oils such as citronella, neroli, cyclamen, lemon grass, tuberose, rose, musk, balsam and tolu. It is used in perfumery to emphasize the odors of sweet floral perfumes. Its method of action for enhancing perfume scent is as a co-solvent that regulates the volatility of the odorants. It is especially used in lilac perfumes.
Farnesol is a natural pesticide for mites and is a pheromone for several other insects.
In a 1994 report released by five top cigarette companies, farnesol was listed as one of 599 additives to cigarettes.[1] It is a flavoring ingredient.
Natural source and synthesis
Farnesol is produced from isoprene compounds in both plants and animals. When geranyl pyrophosphate reacts with isopentenyl pyrophosphate, the result is the 15-carbon farnesyl pyrophosphate, which is an intermediate in the biosynthesis of sesquiterpenes such as farnesene. Oxidation can then provide sesquiterpenoids such as farnesol.
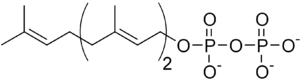
History of the name
Farnesol is found in a flower extract with a long history of use in perfumery. The pure substance farnesol was named (ca. 1900-1905) after the Farnese acacia tree (Vachellia farnesiana), since the flowers from the tree were the commercial source of the floral essence in which the chemical was identified. This particular acacia species, in turn, is named after Cardinal Odoardo Farnese (1573-1626) of the notable Italian Farnese family which (from 1550 though the 17th century) maintained some of the first private European botanical gardens in the Farnese gardens in Rome. The addition of the -ol ending results from it being chemically an alcohol.[2] The plant itself was brought to the Farnese gardens from the Caribbean and Central America, where it originates.[3]
Health effects
Farnesol has been suggested to function as a chemopreventative and anti-tumor agent.[4] Farnesol is used as a deodorant in cosmetic products because of its anti-bacterial activity.[5] Farnesol is subject to restrictions on its use in perfumery[6] as some people may become sensitised to it, however the evidence that farnesol can cause an allergic reaction in humans is disputed.[7]
Biological function
Farnesol is used by the commensal, opportunistically pathogenic fungus Candida albicans as a quorum sensing molecule that inhibits filamentation.[8]
References
- ↑ List of Additives in Cigarettes
- ↑ http://dictionary.reference.com/browse/farnesol Etymology of farnesol, accessed August 27, 2009.
- ↑ HENRY TRIMBLE AND F. D. MACFARLAND., AMERICAN JOURNAL OF PHARMACY, Volume 57, #3, March, 1885
- ↑ Joo JH, Jetten AM (June 2009). "Molecular mechanisms involved in farnesol-induced apoptosis". Cancer Lett. 287 (2): 123–35. doi:10.1016/j.canlet.2009.05.015. PMC 2815016. PMID 19520495.
- ↑ Kromidas, L; Perrier, E; Flanagan, J; Rivero, R; Bonnet, I (2006). "Release of antimicrobial actives from microcapsules by the action of axillary bacteria". Int J Cosmet Sci 28 (2): 103–108. doi:10.1111/j.1467-2494.2006.00283.x. PMID 18492144.
- ↑ http://www.ifraorg.org/en-us/standards_restricted/s3/p4
- ↑ http://www.leffingwell.com/Cropwatch%20Claims%20Victory%20Over%2026%20Allergens.pdf
- ↑ Quorum Sensing in the Dimorphic FungusCandida albicans Is Mediated by Farnesol
See also
- Farnesylation
- Farnesene
- Farnesyl pyrophosphate
- Geranylgeraniol
- Nerolidol