Faldaprevir
 | |
| Systematic (IUPAC) name | |
|---|---|
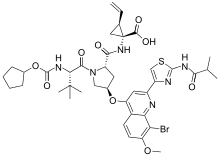
| N-[(Cyclopentyloxy)carbonyl]-3-methyl-L-valyl-(4R)-4-({8-bromo-2-[2-(isobutyrylamino)-1,3-thiazol-4-yl]-7-methoxy-4-quinolinyl}oxy)-N-[(1R,2S)-1-carboxy-2-vinylcyclopropyl]-L-prolinamide | |
| Clinical data | |
| |
| |
| Oral | |
| Identifiers | |
| 801283-95-4 | |
| J05AE13 | |
| ChemSpider | 26327117 |
| Chemical data | |
| Formula | C40H49BrN6O9S |
| 869.82 g/mol | |
|
SMILES
| |
| |
Faldaprevir (formerly BI 201335) is an experimental drug for the treatment of hepatitis C. It is being developed by Boehringer-Ingelheim and is currently in Phase III trials.[1]
Faldaprevir is a hepatitis C virus protease inhibitor.
Faldaprevir is being tested in combination regimens with pegylated interferon and ribavirin, and in interferon-free regimens with other direct-acting antiviral agents including BI 207127.
Data from the SOUND-C2 study, presented at the 2012 AASLD Liver Meeting, showed that a triple combination of faldaprevir, BI 207127, and ribavirin performed well in HCV genotype 1b patients.[2] Efficacy fell below 50%, however, for dual regimens without ribavirin and for genotype 1a patients.
References
- ↑ Efficacy and Safety of BI 201335 (Faldaprevir) in Combination With Pegylated Interferon-alpha and Ribavirin in Treatment-naïve Genotype 1 Hepatitis C Infected Patients (STARTverso 1). Cliicaltrials.gov. March 6, 2013.
- ↑ Interferon-free hepatitis C treatment with faldaprevir proves safe and effective in people with cirrhosis. Alcorn, K. Aidsmap.com. 20 November 2012.
External links
- Faldaprevir at chem.sis.nlm.nih.gov
- Faldaprevir at chemicalregister.com
| ||||||||||||||||||||||||||||||||||||||||||