FLNC (gene)
| Filamin C, gamma | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 1v05. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | FLNC ; ABP-280; ABP280A; ABPA; ABPL; FLN2; MFM5; MPD4 | ||||||||||||
| External IDs | OMIM: 102565 MGI: 95557 HomoloGene: 37481 GeneCards: FLNC Gene | ||||||||||||
| |||||||||||||
| RNA expression pattern | |||||||||||||
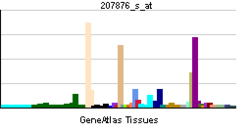 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | 2318 | 68794 | |||||||||||
| Ensembl | ENSG00000128591 | ENSMUSG00000068699 | |||||||||||
| UniProt | Q14315 | Q8VHX6 | |||||||||||
| RefSeq (mRNA) | NM_001127487 | NM_001081185 | |||||||||||
| RefSeq (protein) | NP_001120959 | NP_001074654 | |||||||||||
| Location (UCSC) | Chr 7: 128.47 – 128.5 Mb | Chr 6: 29.43 – 29.46 Mb | |||||||||||
| PubMed search | |||||||||||||
Filamin-C (FLN-C) also known as actin-binding-like protein (ABPL) or filamin-2 (FLN2) is a protein that in humans is encoded by the FLNC gene.[1][2][3] Filamin-C is mainly expressed in cardiac and skeletal muscles, and functions at Z-discs and in subsarcolemmal regions.
Structure
Filamin-C is a 290.8 kDa protein composed of 2725 amino acids.[4][5] Filamin-C, like the ubiquitously-expressed isoform Filamin-A, have an N-terminal filamentous actin-binding domain, followed by a lengthy C-terminal self-association domain containing a series of immunoglobulin-like domains, and a membrane glycoprotein-binding domain.[6] Filamin-C interacts with γ-sarcoglycan and δ-sarcoglycan at the sarcolemma;[7][7][8][8] myotilin and FATZ/calsarcin/myozenin at Z-lines,[9][10][11][12] as well as LL5β.[13] Filamin-C has also been shown to interact with INPPL1,[14] KCND2,[15] and MAP2K4.[16]
Function
The family of Filamin proteins crosslink actin filaments into orthogonal networks in cortical cytoplasm and participate in the anchoring of membrane proteins for the actin cytoskeleton. However, the precise function of the Filamin-C isoform is still under investigation. As Filamin-C is localized mainly to striated muscle, its functions are likely specific to the specialized sarcomeric cytoskeleton present in muscle. As Filamin-C is found at both subsarcolemmal regions and at Z-lines, one plausible function of Filamin-C would be to act as a mode of communication between the membrane and the sarcomere. In skeletal muscle, Filamin-C is found at sites of core formation in skeletal myopathies,[17] and alterations in subcellular localization of Filamin-C have been exhibited in limb-girdle muscular dystrophy and Duchenne muscular dystrophy.[18]
Clinical significance
Mutations in Filamin C have been associated with human hypertrophic cardiomyopathy and a higher incidence of sudden cardiac death.[19] Expression of mutant protein in rat cardiac cells demonstrated that mutant Filamin C forms aggregates, which may provide a mechanistic link to the observed cardiac dysfunction.[19] Deficiency of this protein has been associated with muscle weakness.[20]
References
- ↑ Maestrini E, Patrosso C, Mancini M, Rivella S, Rocchi M, Repetto M et al. (Jun 1993). "Mapping of two genes encoding isoforms of the actin binding protein ABP-280, a dystrophin like protein, to Xq28 and to chromosome 7". Human Molecular Genetics 2 (6): 761–6. doi:10.1093/hmg/2.6.761. PMID 7689010.
- ↑ Gariboldi M, Maestrini E, Canzian F, Manenti G, De Gregorio L, Rivella S et al. (May 1994). "Comparative mapping of the actin-binding protein 280 genes in human and mouse". Genomics 21 (2): 428–30. doi:10.1006/geno.1994.1288. PMID 8088838.
- ↑ "Entrez Gene: FLNC filamin C, gamma (actin binding protein 280)".
- ↑ http://www.heartproteome.org/copa/ProteinInfo.aspx?QType=Protein%20ID&QValue=Q14315. Missing or empty
|title=(help) - ↑ Zong, N. C.; Li, H; Li, H; Lam, M. P.; Jimenez, R. C.; Kim, C. S.; Deng, N; Kim, A. K.; Choi, J. H.; Zelaya, I; Liem, D; Meyer, D; Odeberg, J; Fang, C; Lu, H. J.; Xu, T; Weiss, J; Duan, H; Uhlen, M; Yates Jr, 3rd; Apweiler, R; Ge, J; Hermjakob, H; Ping, P (2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.
- ↑ van der Flier A, Sonnenberg A (2001). "Structural and functional aspects of filamins". Biochim. Biophys. Acta 1538 (2-3): 99–117. doi:10.1016/s0167-4889(01)00072-6. PMID 11336782.
- ↑ 7.0 7.1 Guyon JR, Kudryashova E, Potts A, Dalkilic I, Brosius MA, Thompson TG et al. (Oct 2003). "Calpain 3 cleaves filamin C and regulates its ability to interact with gamma- and delta-sarcoglycans". Muscle & Nerve 28 (4). doi:10.1002/mus.10465. PMID 14506720.
- ↑ 8.0 8.1 Thompson TG, Chan YM, Hack AA, Brosius M, Rajala M, Lidov HG et al. (Jan 2000). "Filamin 2 (FLN2): A muscle-specific sarcoglycan interacting protein". The Journal of Cell Biology 148 (1). PMID 10629222.
- ↑ van der Ven PF, Wiesner S, Salmikangas P, Auerbach D, Himmel M, Kempa S et al. (2000). "Indications for a novel muscular dystrophy pathway. gamma-filamin, the muscle-specific filamin isoform, interacts with myotilin". J. Cell Biol. 151 (2): 235–48. doi:10.1083/jcb.151.2.235. PMC 2192634. PMID 11038172.
- ↑ Takada F, Vander Woude DL, Tong HQ, Thompson TG, Watkins SC, Kunkel LM et al. (Feb 2001). "Myozenin: an alpha-actinin- and gamma-filamin-binding protein of skeletal muscle Z lines". Proceedings of the National Academy of Sciences of the United States of America 98 (4): 1595–600. doi:10.1073/pnas.041609698. PMC 29302. PMID 11171996.
- ↑ Frey N, Olson EN (Apr 2002). "Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins". The Journal of Biological Chemistry 277 (16): 13998–4004. doi:10.1074/jbc.M200712200. PMID 11842093.
- ↑ Faulkner G, Pallavicini A, Comelli A, Salamon M, Bortoletto G, Ievolella C et al. (Dec 2000). "FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle". The Journal of Biological Chemistry 275 (52): 41234–42. doi:10.1074/jbc.M007493200. PMID 10984498.
- ↑ Paranavitane V, Coadwell WJ, Eguinoa A, Hawkins PT, Stephens L (Jan 2003). "LL5beta is a phosphatidylinositol (3,4,5)-trisphosphate sensor that can bind the cytoskeletal adaptor, gamma-filamin". The Journal of Biological Chemistry 278 (2): 1328–35. doi:10.1074/jbc.M208352200. PMID 12376540.
- ↑ Dyson JM, O'Malley CJ, Becanovic J, Munday AD, Berndt MC, Coghill ID et al. (Dec 2001). "The SH2-containing inositol polyphosphate 5-phosphatase, SHIP-2, binds filamin and regulates submembraneous actin". The Journal of Cell Biology 155 (6): 1065–79. doi:10.1083/jcb.200104005. PMC 2150887. PMID 11739414.
- ↑ Petrecca K, Miller DM, Shrier A (Dec 2000). "Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin-binding protein filamin". The Journal of Neuroscience 20 (23): 8736–44. PMID 11102480.
- ↑ Marti A, Luo Z, Cunningham C, Ohta Y, Hartwig J, Stossel TP et al. (Jan 1997). "Actin-binding protein-280 binds the stress-activated protein kinase (SAPK) activator SEK-1 and is required for tumor necrosis factor-alpha activation of SAPK in melanoma cells". The Journal of Biological Chemistry 272 (5): 2620–8. doi:10.1074/jbc.272.5.2620. PMID 9006895.
- ↑ Bönnemann, C. G.; Thompson, T. G.; Van Der Ven, P. F.; Goebel, H. H.; Warlo, I; Vollmers, B; Reimann, J; Herms, J; Gautel, M; Takada, F; Beggs, A. H.; Fürst, D. O.; Kunkel, L. M.; Hanefeld, F; Schröder, R (2003). "Filamin C accumulation is a strong but nonspecific immunohistochemical marker of core formation in muscle". Journal of the neurological sciences 206 (1): 71–8. PMID 12480088.
- ↑ Thompson, T. G.; Chan, Y. M.; Hack, A. A.; Brosius, M; Rajala, M; Lidov, H. G.; McNally, E. M.; Watkins, S; Kunkel, L. M. (2000). "Filamin 2 (FLN2): A muscle-specific sarcoglycan interacting protein". The Journal of cell biology 148 (1): 115–26. PMC 3207142. PMID 10629222.
- ↑ 19.0 19.1 Valdes-Mas R, Gutierrez-Fernandez A, Gomez J, Coto E, Astudillo A, Puente DA et al. "Mutations in filamin C cause a new form of familial hypertrophic cardiomyopathy". Nature Communications 5. doi:10.1038/ncomms6326. PMID 25351925.
- ↑ Guergueltcheva V, Peeters K, Baets J, Ceuterick-de Groote C, Martin JJ, Suls A et al. (Dec 2011). "Distal myopathy with upper limb predominance caused by filamin C haploinsufficiency". Neurology 77 (24): 2105–14. doi:10.1212/WNL.0b013e31823dc51e. PMID 22131542.
Further reading
- Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M et al. (Feb 2001). "Filamins as integrators of cell mechanics and signalling". Nature Reviews. Molecular Cell Biology 2 (2): 138–45. doi:10.1038/35052082. PMID 11252955.
- Lanfranchi G, Muraro T, Caldara F, Pacchioni B, Pallavicini A, Pandolfo D et al. (Jan 1996). "Identification of 4370 expressed sequence tags from a 3'-end-specific cDNA library of human skeletal muscle by DNA sequencing and filter hybridization". Genome Research 6 (1): 35–42. doi:10.1101/gr.6.1.35. PMID 8681137.
- Marti A, Luo Z, Cunningham C, Ohta Y, Hartwig J, Stossel TP et al. (Jan 1997). "Actin-binding protein-280 binds the stress-activated protein kinase (SAPK) activator SEK-1 and is required for tumor necrosis factor-alpha activation of SAPK in melanoma cells". The Journal of Biological Chemistry 272 (5): 2620–8. doi:10.1074/jbc.272.5.2620. PMID 9006895.
- Liu G, Thomas L, Warren RA, Enns CA, Cunningham CC, Hartwig JH et al. (Dec 1997). "Cytoskeletal protein ABP-280 directs the intracellular trafficking of furin and modulates proprotein processing in the endocytic pathway". The Journal of Cell Biology 139 (7): 1719–33. doi:10.1083/jcb.139.7.1719. PMC 1424222. PMID 9412467.
- Xu W, Xie Z, Chung DW, Davie EW (Aug 1998). "A novel human actin-binding protein homologue that binds to platelet glycoprotein Ibalpha". Blood 92 (4): 1268–76. PMID 9694715.
- Xie Z, Xu W, Davie EW, Chung DW (Oct 1998). "Molecular cloning of human ABPL, an actin-binding protein homologue". Biochemical and Biophysical Research Communications 251 (3): 914–9. doi:10.1006/bbrc.1998.9506. PMID 9791010.
- Thompson TG, Chan YM, Hack AA, Brosius M, Rajala M, Lidov HG et al. (Jan 2000). "Filamin 2 (FLN2): A muscle-specific sarcoglycan interacting protein". The Journal of Cell Biology 148 (1): 115–26. doi:10.1083/jcb.148.1.115. PMC 3207142. PMID 10629222.
- van der Ven PF, Obermann WM, Lemke B, Gautel M, Weber K, Fürst DO (Feb 2000). "Characterization of muscle filamin isoforms suggests a possible role of gamma-filamin/ABP-L in sarcomeric Z-disc formation". Cell Motility and the Cytoskeleton 45 (2): 149–62. doi:10.1002/(SICI)1097-0169(200002)45:2<149::AID-CM6>3.0.CO;2-G. PMID 10658210.
- Faulkner G, Pallavicini A, Comelli A, Salamon M, Bortoletto G, Ievolella C et al. (Dec 2000). "FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle". The Journal of Biological Chemistry 275 (52): 41234–42. doi:10.1074/jbc.M007493200. PMID 10984498.
- van der Ven PF, Wiesner S, Salmikangas P, Auerbach D, Himmel M, Kempa S et al. (Oct 2000). "Indications for a novel muscular dystrophy pathway. gamma-filamin, the muscle-specific filamin isoform, interacts with myotilin". The Journal of Cell Biology 151 (2): 235–48. doi:10.1083/jcb.151.2.235. PMC 2192634. PMID 11038172.
- Petrecca K, Miller DM, Shrier A (Dec 2000). "Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin-binding protein filamin". The Journal of Neuroscience 20 (23): 8736–44. PMID 11102480.
- Chakarova C, Wehnert MS, Uhl K, Sakthivel S, Vosberg HP, van der Ven PF et al. (Dec 2000). "Genomic structure and fine mapping of the two human filamin gene paralogues FLNB and FLNC and comparative analysis of the filamin gene family". Human Genetics 107 (6): 597–611. doi:10.1007/s004390000414. PMID 11153914.
- Takada F, Vander Woude DL, Tong HQ, Thompson TG, Watkins SC, Kunkel LM et al. (Feb 2001). "Myozenin: an alpha-actinin- and gamma-filamin-binding protein of skeletal muscle Z lines". Proceedings of the National Academy of Sciences of the United States of America 98 (4): 1595–600. doi:10.1073/pnas.041609698. PMC 29302. PMID 11171996.
- Dyson JM, O'Malley CJ, Becanovic J, Munday AD, Berndt MC, Coghill ID et al. (Dec 2001). "The SH2-containing inositol polyphosphate 5-phosphatase, SHIP-2, binds filamin and regulates submembraneous actin". The Journal of Cell Biology 155 (6): 1065–79. doi:10.1083/jcb.200104005. PMC 2150887. PMID 11739414.
- Frey N, Olson EN (Apr 2002). "Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins". The Journal of Biological Chemistry 277 (16): 13998–4004. doi:10.1074/jbc.M200712200. PMID 11842093.
- Donaldson JC, Dise RS, Ritchie MD, Hanks SK (Aug 2002). "Nephrocystin-conserved domains involved in targeting to epithelial cell-cell junctions, interaction with filamins, and establishing cell polarity". The Journal of Biological Chemistry 277 (32): 29028–35. doi:10.1074/jbc.M111697200. PMID 12006559.
- Shoeman RL, Hartig R, Hauses C, Traub P (2003). "Organization of focal adhesion plaques is disrupted by action of the HIV-1 protease". Cell Biology International 26 (6): 529–39. doi:10.1006/cbir.2002.0895. PMID 12119179.
External links
- Mass spectrometry characterization of human FLNC at COPaKB [1]
- GeneReviews/NIH/NCBI/UW entry on Myofibrillar Myopathy
| |||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||