Eyring equation
The Eyring equation (occasionally also known as Eyring–Polanyi equation) is an equation used in chemical kinetics to describe the variance of the rate of a chemical reaction with temperature. It was developed almost simultaneously in 1935 by Henry Eyring, Meredith Gwynne Evans and Michael Polanyi. This equation follows from the transition state theory (aka, activated-complex theory) and is trivially equivalent to the empirical Arrhenius equation which are both readily derived from statistical thermodynamics in the kinetic theory of gases.[1]
General form
The general form of the Eyring–Polanyi equation somewhat resembles the Arrhenius equation:
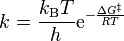
where ΔG‡ is the Gibbs energy of activation, kB is Boltzmann's constant, and h is Planck's constant.
It can be rewritten as:
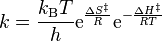
To find the linear form of the Eyring-Polanyi equation:
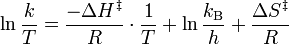
where:
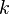 = reaction rate constant
= reaction rate constant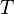 = absolute temperature
= absolute temperature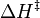 = enthalpy of activation
= enthalpy of activation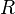 = gas constant
= gas constant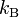 = Boltzmann constant
= Boltzmann constant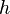 = Planck's constant
= Planck's constant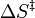 = entropy of activation
= entropy of activation
A certain chemical reaction is performed at different temperatures and the reaction rate is determined. The plot of 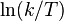 versus
versus 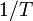 gives a straight line with slope
gives a straight line with slope 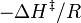 from which the enthalpy of activation can be derived and with intercept
from which the enthalpy of activation can be derived and with intercept 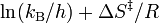 from which the entropy of activation is derived.
from which the entropy of activation is derived.
Accuracy
Transition state theory requires a value of a certain transmission coefficient, called 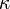 in that theory, as an additional prefactor in the Eyring equation above. This value is usually taken to be unity (i.e., the transition state
in that theory, as an additional prefactor in the Eyring equation above. This value is usually taken to be unity (i.e., the transition state  always proceeds to products
always proceeds to products 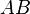 and never reverts to reactants
and never reverts to reactants 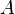 and
and 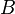 ), and we have followed this convention above. Alternatively, to avoid specifying a value of
), and we have followed this convention above. Alternatively, to avoid specifying a value of 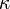 , the ratios of rate constants can be compared to the value of a rate constant at some fixed reference temperature (i.e.,
, the ratios of rate constants can be compared to the value of a rate constant at some fixed reference temperature (i.e., 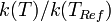 ) which eliminates the
) which eliminates the  term in the resulting expression.
term in the resulting expression.
Notes
- ↑ Chapman & Enskog 1939
References
- Evans, M.G.; Polanyi M. (1935). "Some applications of the transition state method to the calculation of reaction velocities, especially in solution". Trans. Faraday Soc. 31: 875–894. doi:10.1039/tf9353100875.
- Eyring, H. (1935). "The Activated Complex in Chemical Reactions". J. Chem. Phys. 3 (2): 107–115. Bibcode:1935JChPh...3..107E. doi:10.1063/1.1749604.
- Eyring, H.; Polanyi M. (1931). "Über Einfache Gasreaktionen". Z. Phys. Chem. Abt. B 12: 279–311.
- Laidler, K.J.; King M.C. (1983). "The development of Transition-State Theory". J. Phys. Chem. 87 (15): 2657–2664. doi:10.1021/j100238a002.
- Polanyi, J.C. (1987). "Some concepts in reaction dynamics". Science 236 (4802): 680–690. Bibcode:1987Sci...236..680P. doi:10.1126/science.236.4802.680.
- Chapman, S. and Cowling, T.G. (1991). "The Mathematical Theory of Non-uniform Gases: An Account of the Kinetic Theory of Viscosity, Thermal Conduction and Diffusion in Gases" (3rd Edition). Cambridge University Press, ISBN 9780521408448