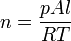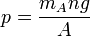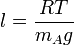Exosphere

The exosphere (Ancient Greek: ἔξω éxō "outside, external, beyond", Ancient Greek: σφαῖρα sphaĩra "sphere") is a thin, atmosphere-like volume surrounding a planetary body where molecules are gravitationally bound to that body, but where the density is too low for them to behave as a gas by colliding with each other. In the case of bodies with substantial atmospheres, such as Earth's atmosphere, the exosphere is the uppermost layer, where the atmosphere thins out and merges with interplanetary space. It is located directly above the thermosphere.
Several moons, such as the Moon and the Galilean satellites, have exospheres without a denser atmosphere underneath.[1] Here, molecules are ejected on parabolic trajectories until they collide with the surface. Smaller bodies such as asteroids, in which the molecules emitted from the surface escape to space, are not considered to have exospheres.
Earth's exosphere
The main gases within Earth's exosphere are the lightest atmospheric gases, mainly hydrogen, with some helium, carbon dioxide, and atomic oxygen near the base of the exosphere. Because there is no clear boundary between outer space and the exosphere, the exosphere is sometimes considered a part of outer space.
Lower boundary
The lower boundary of the exosphere is known as exopause; it is also called the exobase, as in Earth's atmosphere the atmospheric temperature becomes nearly a constant above this altitude. Before the term exobase was established the boundary was also called the critical altitude where barometric conditions no longer apply.[2] The altitude of the exobase ranges from about 500 to 1,000 kilometres (310 to 620 mi) depending on solar activity.[3]
The exobase can be defined in one of two ways:
If we define the exobase as the height at which upward-traveling molecules experience one collision on average, then at this position the mean free path of a molecule is equal to one pressure scale height. This is shown in the following. Consider a volume of air, with horizontal area 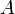 and height equal to the mean free path
and height equal to the mean free path 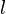 , at pressure
, at pressure 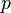 and temperature
and temperature 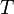 . For an ideal gas, the number of molecules contained in it is:
. For an ideal gas, the number of molecules contained in it is:
where 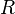 is the universal gas constant. From the requirement that each molecule traveling upward undergoes on average one collision, the pressure is:
is the universal gas constant. From the requirement that each molecule traveling upward undergoes on average one collision, the pressure is:
where 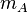 is the mean molecular mass of the gas. Solving these two equations gives:
is the mean molecular mass of the gas. Solving these two equations gives:
which is the equation for the pressure scale height. As the pressure scale height is almost equal to the density scale height of the primary constituent, and because the Knudsen number is the ratio of mean free path and typical density fluctuation scale, this means that the exobase lies in the region where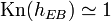 .
.
The fluctuation in the height of the exobase is important because this provides atmospheric drag on satellites, eventually causing them to fall from orbit if no action is taken to maintain the orbit.
Upper boundary
In principle, the exosphere covers distances where particles are still gravitationally bound to Earth, i.e. particles still have ballistic orbits that will take them back towards Earth. The upper boundary of the exosphere can be defined as the distance at which the influence of solar radiation pressure on atomic hydrogen exceeds that of Earth's gravitational pull. This happens at half the distance to the Moon (190,000 kilometres (120,000 mi)). The exosphere, observable from space as the geocorona, is seen to extend to at least 10,000 kilometres (6,200 mi) from Earth's surface. The exosphere is a transitional zone between Earth's atmosphere and interplanetary space.
References
- ↑ Day, Brian (August 20, 2013). "Why LADEE Matters". NASA Ames Research Center. Retrieved April 19, 2015.
- ↑ Bauer & Lammer, Planetary Aeronomy: Atmosphere Environments in Planetary Systems, Springer, 2004.
- ↑ "Exosphere - overview". UCAR. 2011. Retrieved April 19, 2015.
External links
- Gerd W. Prolss: Physics of the Earth's Space Environment: An Introduction. ISBN 3-540-21426-7
| ||||||||||||||