Evolutionary history of life
| Part of a series on |
| Evolutionary biology |
|---|
 Diagrammatic representation of the divergence of modern taxonomic groups from their common ancestor. |
|
Natural history
|
|
History of evolutionary theory |
|
Fields and applications
|
|
Social implications |
|
The evolutionary history of life on Earth traces the processes by which living and fossil organisms have evolved since life on the planet first originated until the present day. Earth formed about 4.5 Ga (billion years ago) and life appeared on its surface within 1 billion years. The similarities between all present-day organisms indicate the presence of a common ancestor from which all known species have diverged through the process of evolution.[1] Although more than 99 percent of all species that ever lived on the planet are estimated to be extinct,[2][3] there are currently 10–14 million species of life on the Earth.[4]
The earliest evidence for life on Earth is graphite found to be biogenic in 3.7 billion-year-old metasedimentary rocks discovered in western Greenland[5] and microbial mat fossils found in 3.48 billion-year-old sandstone discovered in Western Australia.[6][7] Microbial mats of coexisting bacteria and archaea were the dominant form of life in the early Archean and many of the major steps in early evolution are thought to have taken place within them.[8] The evolution of oxygenic photosynthesis, around 3.5 Ga, eventually led to the oxygenation of the atmosphere, beginning around 2.4 Ga.[9] The earliest evidence of eukaryotes (complex cells with organelles) dates from 1.85 Ga,[10][11] and while they may have been present earlier, their diversification accelerated when they started using oxygen in their metabolism. Later, around 1.7 Ga, multicellular organisms began to appear, with differentiated cells performing specialised functions.[12] Bilateria, animals with a front and a back, appeared by 555 Ma (million years ago).[13]
The earliest land plants date back to around 450 Ma,[14] although evidence suggests that microorganisms formed the earliest terrestrial ecosystems, at least 2.9 Ga.[15] Microorganisms are thought to have paved the way for the inception of land plants in the Phanerozoic. Land plants were so successful that they are thought to have contributed to the Late Devonian extinction event.[16] Invertebrate animals appear during the Ediacaran period,[17] while vertebrates originated about 525 Ma during the Cambrian explosion.[18] During the Permian period, synapsids, including the ancestors of mammals, dominated the land,[19] but most of this group became extinct in the Permian–Triassic extinction event 252.17 Ma.[20] During the recovery from this catastrophe, archosaurs became the most abundant land vertebrates;[21] one archosaur group, the dinosaurs, dominated the Jurassic and Cretaceous periods.[22] After the Cretaceous–Paleogene extinction event 66 Ma killed off the dinosaurs,[23] mammals increased rapidly in size and diversity.[24] Such mass extinctions may have accelerated evolution by providing opportunities for new groups of organisms to diversify.[25]
Earliest history of Earth
Ma (Millions of years)
The oldest meteorite fragments found on Earth are about 4.54 billion years old; this, coupled primarily with the dating of ancient lead deposits, has put the estimated age of Earth at around that time.[27] The Moon has the same composition as Earth's crust but does not contain an iron-rich core like the Earth's. Many scientists think that about 40 million years later a body the size of Mars struck the Earth, throwing into orbit crust material that formed the Moon. Another hypothesis is that the Earth and Moon started to coalesce at the same time but the Earth, having much stronger gravity than the early Moon, attracted almost all the iron particles in the area.[28]
Until 2001, the oldest rocks found on Earth were about 3.8 billion years old,[29][30][31][32] leading scientists to estimate that the Earth's surface had been molten until then. Accordingly, they named this part of Earth's history the Hadean, whose name means "hellish."[33] However, analysis of zircons formed 4.4 Ga indicates that Earth's crust solidified about 100 million years after the planet's formation and that the planet quickly acquired oceans and an atmosphere, which may have been capable of supporting life.[34][35]
Evidence from the Moon indicates that from 4 to 3.8 Ga it suffered a Late Heavy Bombardment by debris that was left over from the formation of the Solar System, and the Earth should have experienced an even heavier bombardment due to its stronger gravity.[33][36] While there is no direct evidence of conditions on Earth 4 to 3.8 Ga, there is no reason to think that the Earth was not also affected by this late heavy bombardment.[37] This event may well have stripped away any previous atmosphere and oceans; in this case gases and water from comet impacts may have contributed to their replacement, although volcanic outgassing on Earth would have supplied at least half.[38] However, if subsurface microbial life had evolved by this point, it would have survived the bombardment.[39]
Earliest evidence for life on Earth
The earliest identified organisms were minute and relatively featureless, and their fossils look like small rods, which are very difficult to tell apart from structures that arise through abiotic physical processes. The oldest undisputed evidence of life on Earth, interpreted as fossilized bacteria, dates to 3 Ga.[40] Other finds in rocks dated to about 3.5 Ga have been interpreted as bacteria,[41] with geochemical evidence also seeming to show the presence of life 3.8 Ga.[42] However, these analyses were closely scrutinized, and non-biological processes were found which could produce all of the "signatures of life" that had been reported.[43][44] While this does not prove that the structures found had a non-biological origin, they cannot be taken as clear evidence for the presence of life. Geochemical signatures from rocks deposited 3.4 Ga have been interpreted as evidence for life,[40][45] although these statements have not been thoroughly examined by critics.
Origins of life on Earth

- ^ Ciccarelli, Francesca D.; Doerks, Tobias; von Mering, Christian et al. (March 3, 2006). "Toward Automatic Reconstruction of a Highly Resolved Tree of Life". Science (Washington, D.C.: American Association for the Advancement of Science) 311 (5765): 1283–1287. Bibcode:2006Sci...311.1283C. doi:10.1126/science.1123061. ISSN 0036-8075. PMID 16513982.
Biologists reason that all living organisms on Earth must share a single last universal ancestor, because it would be virtually impossible that two or more separate lineages could have independently developed the many complex biochemical mechanisms common to all living organisms.[46][47] As previously mentioned the earliest organisms for which fossil evidence is available are bacteria. The lack of fossil or geochemical evidence for earlier organisms has left plenty of scope for hypotheses, which fall into two main groups: 1) that life arose spontaneously on Earth or 2) that it was "seeded" from elsewhere in the Universe.
Life "seeded" from elsewhere
The idea that life on Earth was "seeded" from elsewhere in the Universe dates back at least to the Greek philosopher Anaximander in the sixth century BCE.[48] In the twentieth century it was proposed by the physical chemist Svante Arrhenius,[49] by the astronomers Fred Hoyle and Chandra Wickramasinghe,[50] and by molecular biologist Francis Crick and chemist Leslie Orgel.[51] There are three main versions of the "seeded from elsewhere" hypothesis: from elsewhere in our Solar System via fragments knocked into space by a large meteor impact, in which case the most credible sources are Mars[52] and Venus;[53] by alien visitors, possibly as a result of accidental contamination by microorganisms that they brought with them;[51] and from outside the Solar System but by natural means.[49][52] Experiments in low Earth orbit, such as EXOSTACK, demonstrated that some microorganism spores can survive the shock of being catapulted into space and some can survive exposure to outer space radiation for at least 5.7 years.[54][55] Scientists are divided over the likelihood of life arising independently on Mars,[56] or on other planets in our galaxy.[52]
Independent emergence on Earth
Life on Earth is based on carbon and water. Carbon provides stable frameworks for complex chemicals and can be easily extracted from the environment, especially from carbon dioxide. The only other element with similar chemical properties, silicon, forms much less stable structures and, because most of its compounds are solids, would be more difficult for organisms to extract. Water is an excellent solvent and has two other useful properties: the fact that ice floats enables aquatic organisms to survive beneath it in winter; and its molecules have electrically negative and positive ends, which enables it to form a wider range of compounds than other solvents can. Other good solvents, such as ammonia, are liquid only at such low temperatures that chemical reactions may be too slow to sustain life, and lack water's other advantages.[57] Organisms based on alternative biochemistry may, however, be possible on other planets.[58]
Research on how life might have emerged from non-living chemicals focuses on three possible starting points: self-replication, an organism's ability to produce offspring that are very similar to itself; metabolism, its ability to feed and repair itself; and external cell membranes, which allow food to enter and waste products to leave, but exclude unwanted substances.[59] Research on abiogenesis still has a long way to go, since theoretical and empirical approaches are only beginning to make contact with each other.[60][61]
Replication first: RNA world
Even the simplest members of the three modern domains of life use DNA to record their "recipes" and a complex array of RNA and protein molecules to "read" these instructions and use them for growth, maintenance and self-replication. The discovery that some RNA molecules can catalyze both their own replication and the construction of proteins led to the hypothesis of earlier life-forms based entirely on RNA.[62] These ribozymes could have formed an RNA world in which there were individuals but no species, as mutations and horizontal gene transfers would have meant that the offspring in each generation were quite likely to have different genomes from those that their parents started with.[63] RNA would later have been replaced by DNA, which is more stable and therefore can build longer genomes, expanding the range of capabilities a single organism can have.[63][64][65] Ribozymes remain as the main components of ribosomes, modern cells' "protein factories."[66]
Although short self-replicating RNA molecules have been artificially produced in laboratories,[67] doubts have been raised about where natural non-biological synthesis of RNA is possible.[68] The earliest "ribozymes" may have been formed of simpler nucleic acids such as PNA, TNA or GNA, which would have been replaced later by RNA.[69][70]
In 2003, it was proposed that porous metal sulfide precipitates would assist RNA synthesis at about 100 °C (212 °F) and ocean-bottom pressures near hydrothermal vents. Under this hypothesis, lipid membranes would be the last major cell components to appear and, until then, the protocells would be confined to the pores.[71]
Metabolism first: Iron–sulfur world
A series of experiments starting in 1997 showed that early stages in the formation of proteins from inorganic materials including carbon monoxide and hydrogen sulfide could be achieved by using iron sulfide and nickel sulfide as catalysts. Most of the steps required temperatures of about 100 °C (212 °F) and moderate pressures, although one stage required 250 °C (482 °F) and a pressure equivalent to that found under 7 kilometres (4.3 mi) of rock. Hence it was suggested that self-sustaining synthesis of proteins could have occurred near hydrothermal vents.[72]
Membranes first: Lipid world

It has been suggested that double-walled "bubbles" of lipids like those that form the external membranes of cells may have been an essential first step.[73] Experiments that simulated the conditions of the early Earth have reported the formation of lipids, and these can spontaneously form liposomes, double-walled "bubbles," and then reproduce themselves. Although they are not intrinsically information-carriers as nucleic acids are, they would be subject to natural selection for longevity and reproduction. Nucleic acids such as RNA might then have formed more easily within the liposomes than they would have outside.[74]
The clay hypothesis
RNA is complex and there are doubts about whether it can be produced non-biologically in the wild.[68] Some clays, notably montmorillonite, have properties that make them plausible accelerators for the emergence of an RNA world: they grow by self-replication of their crystalline pattern; they are subject to an analog of natural selection, as the clay "species" that grows fastest in a particular environment rapidly becomes dominant; and they can catalyze the formation of RNA molecules.[75] Although this idea has not become the scientific consensus, it still has active supporters.[76]
Research in 2003 reported that montmorillonite could also accelerate the conversion of fatty acids into "bubbles," and that the "bubbles" could encapsulate RNA attached to the clay. These "bubbles" can then grow by absorbing additional lipids and then divide. The formation of the earliest cells may have been aided by similar processes.[77]
A similar hypothesis presents self-replicating iron-rich clays as the progenitors of nucleotides, lipids and amino acids.[78]
Environmental and evolutionary impact of microbial mats

Microbial mats are multi-layered, multi-species colonies of bacteria and other organisms that are generally only a few millimeters thick, but still contain a wide range of chemical environments, each of which favors a different set of microorganisms.[79] To some extent each mat forms its own food chain, as the by-products of each group of microorganisms generally serve as "food" for adjacent groups.[80]
Stromatolites are stubby pillars built as microorganisms in mats slowly migrate upwards to avoid being smothered by sediment deposited on them by water.[79] There has been vigorous debate about the validity of alleged fossils from before 3 Ga,[81] with critics arguing that so-called stromatolites could have been formed by non-biological processes.[43] In 2006, another find of stromatolites was reported from the same part of Australia as previous ones, in rocks dated to 3.5 Ga.[82]
In modern underwater mats the top layer often consists of photosynthesizing cyanobacteria which create an oxygen-rich environment, while the bottom layer is oxygen-free and often dominated by hydrogen sulfide emitted by the organisms living there.[80] It is estimated that the appearance of oxygenic photosynthesis by bacteria in mats increased biological productivity by a factor of between 100 and 1,000. The reducing agent used by oxygenic photosynthesis is water, which is much more plentiful than the geologically produced reducing agents required by the earlier non-oxygenic photosynthesis.[83] From this point onwards life itself produced significantly more of the resources it needed than did geochemical processes.[84] Oxygen is toxic to organisms that are not adapted to it, but greatly increases the metabolic efficiency of oxygen-adapted organisms.[85][86] Oxygen became a significant component of Earth's atmosphere about 2.4 Ga.[87] Although eukaryotes may have been present much earlier,[88][89] the oxygenation of the atmosphere was a prerequisite for the evolution of the most complex eukaryotic cells, from which all multicellular organisms are built.[90] The boundary between oxygen-rich and oxygen-free layers in microbial mats would have moved upwards when photosynthesis shut down overnight, and then downwards as it resumed on the next day. This would have created selection pressure for organisms in this intermediate zone to acquire the ability to tolerate and then to use oxygen, possibly via endosymbiosis, where one organism lives inside another and both of them benefit from their association.[8]
Cyanobacteria have the most complete biochemical "toolkits" of all the mat-forming organisms. Hence they are the most self-sufficient of the mat organisms and were well-adapted to strike out on their own both as floating mats and as the first of the phytoplankton, providing the basis of most marine food chains.[8]
Diversification of eukaryotes
Chromatin, nucleus, endomembrane system, and mitochondria
Eukaryotes may have been present long before the oxygenation of the atmosphere,[88] but most modern eukaryotes require oxygen, which their mitochondria use to fuel the production of ATP, the internal energy supply of all known cells.[90] In the 1970s it was proposed and, after much debate, widely accepted that eukaryotes emerged as a result of a sequence of endosymbiosis between "prokaryotes." For example: a predatory microorganism invaded a large prokaryote, probably an archaean, but the attack was neutralized, and the attacker took up residence and evolved into the first of the mitochondria; one of these chimeras later tried to swallow a photosynthesizing cyanobacterium, but the victim survived inside the attacker and the new combination became the ancestor of plants; and so on. After each endosymbiosis began, the partners would have eliminated unproductive duplication of genetic functions by re-arranging their genomes, a process which sometimes involved transfer of genes between them.[93][94][95] Another hypothesis proposes that mitochondria were originally sulfur- or hydrogen-metabolising endosymbionts, and became oxygen-consumers later.[96] On the other hand mitochondria might have been part of eukaryotes' original equipment.[97]
There is a debate about when eukaryotes first appeared: the presence of steranes in Australian shales may indicate that eukaryotes were present 2.7 Ga;[89] however, an analysis in 2008 concluded that these chemicals infiltrated the rocks less than 2.2 Ga and prove nothing about the origins of eukaryotes.[98] Fossils of the algae Grypania have been reported in 1.85 billion-year-old rocks (originally dated to 2.1 Ga but later revised[11]), and indicates that eukaryotes with organelles had already evolved.[99] A diverse collection of fossil algae were found in rocks dated between 1.5 and 1.4 Ga.[100] The earliest known fossils of fungi date from 1.43 Ga.[101]
Plastids
Plastids are thought to have originated from endosymbiotic cyanobacteria. The symbiosis evolved around 1.5 Ga and enabled eukaryotes to carry out oxygenic photosynthesis.[90] Three evolutionary lineages have since emerged in which the plastids are named differently: chloroplasts in green algae and plants, rhodoplasts in red algae and cyanelles in the glaucophytes.
Sexual reproduction and multicellular organisms
Evolution of sexual reproduction
The defining characteristics of sexual reproduction in eukaryotes are meiosis and fertilization. There is much genetic recombination in this kind of reproduction, in which offspring receive 50% of their genes from each parent,[102] in contrast with asexual reproduction, in which there is no recombination. Bacteria also exchange DNA by bacterial conjugation, the benefits of which include resistance to antibiotics and other toxins, and the ability to utilize new metabolites.[103] However, conjugation is not a means of reproduction, and is not limited to members of the same species – there are cases where bacteria transfer DNA to plants and animals.[104]
On the other hand, bacterial transformation is clearly an adaptation for transfer of DNA between bacteria of the same species. Bacterial transformation is a complex process involving the products of numerous bacterial genes and can be regarded as a bacterial form of sex.[105][106] This process occurs naturally in at least 67 prokaryotic species (in seven different phyla).[107] Sexual reproduction in eukaryotes may have evolved from bacterial transformation.[108] (Also see Evolution of sexual reproduction#Origin of sexual reproduction.)
The disadvantages of sexual reproduction are well-known: the genetic reshuffle of recombination may break up favorable combinations of genes; and since males do not directly increase the number of offspring in the next generation, an asexual population can out-breed and displace in as little as 50 generations a sexual population that is equal in every other respect.[102] Nevertheless, the great majority of animals, plants, fungi and protists reproduce sexually. There is strong evidence that sexual reproduction arose early in the history of eukaryotes and that the genes controlling it have changed very little since then.[109] How sexual reproduction evolved and survived is an unsolved puzzle.[110]
The Red Queen hypothesis suggests that sexual reproduction provides protection against parasites, because it is easier for parasites to evolve means of overcoming the defenses of genetically identical clones than those of sexual species that present moving targets, and there is some experimental evidence for this. However, there is still doubt about whether it would explain the survival of sexual species if multiple similar clone species were present, as one of the clones may survive the attacks of parasites for long enough to out-breed the sexual species.[102] Furthermore, contrary to the expectations of the Red Queen hypothesis, Kathryn A. Hanley et al. found that the prevalence, abundance and mean intensity of mites was significantly higher in sexual geckos than in asexuals sharing the same habitat.[111] In addition, biologist Matthew Parker, after reviewing numerous genetic studies on plant disease resistance, failed to find a single example consistent with the concept that pathogens are the primary selective agent responsible for sexual reproduction in the host.[112]
Alexey Kondrashov's deterministic mutation hypothesis (DMH) assumes that each organism has more than one harmful mutation and the combined effects of these mutations are more harmful than the sum of the harm done by each individual mutation. If so, sexual recombination of genes will reduce the harm that bad mutations do to offspring and at the same time eliminate some bad mutations from the gene pool by isolating them in individuals that perish quickly because they have an above-average number of bad mutations. However, the evidence suggests that the DMH's assumptions are shaky, because many species have on average less than one harmful mutation per individual and no species that has been investigated shows evidence of synergy between harmful mutations.[102] (Further criticisms of this hypothesis are discussed in the article Evolution of sexual reproduction#Removal of deleterious genes)

The random nature of recombination causes the relative abundance of alternative traits to vary from one generation to another. This genetic drift is insufficient on its own to make sexual reproduction advantageous, but a combination of genetic drift and natural selection may be sufficient. When chance produces combinations of good traits, natural selection gives a large advantage to lineages in which these traits become genetically linked. On the other hand, the benefits of good traits are neutralized if they appear along with bad traits. Sexual recombination gives good traits the opportunities to become linked with other good traits, and mathematical models suggest this may be more than enough to offset the disadvantages of sexual reproduction.[110] Other combinations of hypotheses that are inadequate on their own are also being examined.[102]
The adaptive function of sex today remains a major unresolved issue in biology. The competing models to explain the adaptive function of sex were reviewed by John A. Birdsell and Christopher Wills.[114] The hypotheses discussed above all depend on possible beneficial effects of random genetic variation produced by genetic recombination. An alternative view is that sex arose, and is maintained, as a process for repairing DNA damage, and that the genetic variation produced is an occasionally beneficial byproduct.[108][115]
Multicellularity
The simplest definitions of "multicellular," for example "having multiple cells," could include colonial cyanobacteria like Nostoc. Even a professional biologist's definition such as "having the same genome but different types of cell" would still include some genera of the green algae Volvox, which have cells that specialize in reproduction.[116] Multicellularity evolved independently in organisms as diverse as sponges and other animals, fungi, plants, brown algae, cyanobacteria, slime molds and myxobacteria.[11][117] For the sake of brevity, this article focuses on the organisms that show the greatest specialization of cells and variety of cell types, although this approach to the evolution of biological complexity could be regarded as "rather anthropocentric."[12]

The initial advantages of multicellularity may have included: more efficient sharing of nutrients that are digested outside the cell,[119] increased resistance to predators, many of which attacked by engulfing; the ability to resist currents by attaching to a firm surface; the ability to reach upwards to filter-feed or to obtain sunlight for photosynthesis;[120] the ability to create an internal environment that gives protection against the external one;[12] and even the opportunity for a group of cells to behave "intelligently" by sharing information.[118] These features would also have provided opportunities for other organisms to diversify, by creating more varied environments than flat microbial mats could.[120]
Multicellularity with differentiated cells is beneficial to the organism as a whole but disadvantageous from the point of view of individual cells, most of which lose the opportunity to reproduce themselves. In an asexual multicellular organism, rogue cells which retain the ability to reproduce may take over and reduce the organism to a mass of undifferentiated cells. Sexual reproduction eliminates such rogue cells from the next generation and therefore appears to be a prerequisite for complex multicellularity.[120]
The available evidence indicates that eukaryotes evolved much earlier but remained inconspicuous until a rapid diversification around 1 Ga. The only respect in which eukaryotes clearly surpass bacteria and archaea is their capacity for variety of forms, and sexual reproduction enabled eukaryotes to exploit that advantage by producing organisms with multiple cells that differed in form and function.[120]
Fossil evidence
The Francevillian biota fossils, dated to 2.1 Ga, are the earliest known fossil organisms that are clearly multicellular.[26] They may have had differentiated cells.[121] Another early multicellular fossil, Qingshania,[note 1] dated to 1.7 Ga, appears to consist of virtually identical cells. The red algae called Bangiomorpha, dated at 1.2 Ga, is the earliest known organism that certainly has differentiated, specialized cells, and is also the oldest known sexually reproducing organism.[120] The 1.43 billion-year-old fossils interpreted as fungi appear to have been multicellular with differentiated cells.[101] The "string of beads" organism Horodyskia, found in rocks dated from 1.5 Ga to 900 Ma, may have been an early metazoan;[11] however, it has also been interpreted as a colonial foraminiferan.[113]
Emergence of animals
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
A family tree of the animals[122]
Animals are multicellular eukaryotes,[note 2] and are distinguished from plants, algae, and fungi by lacking cell walls.[123] All animals are motile,[124] if only at certain life stages. All animals except sponges have bodies differentiated into separate tissues, including muscles, which move parts of the animal by contracting, and nerve tissue, which transmits and processes signals.[125]
The earliest widely accepted animal fossils are the rather modern-looking cnidarians (the group that includes jellyfish, sea anemones and Hydra), possibly from around 580 Ma, although fossils from the Doushantuo Formation can only be dated approximately. Their presence implies that the cnidarian and bilaterian lineages had already diverged.[126]
The Ediacara biota, which flourished for the last 40 million years before the start of the Cambrian,[127] were the first animals more than a very few centimeters long. Many were flat and had a "quilted" appearance, and seemed so strange that there was a proposal to classify them as a separate kingdom, Vendozoa.[128] Others, however, been interpreted as early molluscs (Kimberella[129][130]), echinoderms (Arkarua[131]), and arthropods (Spriggina,[132] Parvancorina[133]). There is still debate about the classification of these specimens, mainly because the diagnostic features which allow taxonomists to classify more recent organisms, such as similarities to living organisms, are generally absent in the Ediacarans. However, there seems little doubt that Kimberella was at least a triploblastic bilaterian animal, in other words, an animal significantly more complex than the cnidarians.[134]
The small shelly fauna are a very mixed collection of fossils found between the Late Ediacaran and Middle Cambrian periods. The earliest, Cloudina, shows signs of successful defense against predation and may indicate the start of an evolutionary arms race. Some tiny Early Cambrian shells almost certainly belonged to molluscs, while the owners of some "armor plates," Halkieria and Microdictyon, were eventually identified when more complete specimens were found in Cambrian lagerstätten that preserved soft-bodied animals.[135]
In the 1970s there was already a debate about whether the emergence of the modern phyla was "explosive" or gradual but hidden by the shortage of Precambrian animal fossils.[135] A re-analysis of fossils from the Burgess Shale lagerstätte increased interest in the issue when it revealed animals, such as Opabinia, which did not fit into any known phylum. At the time these were interpreted as evidence that the modern phyla had evolved very rapidly in the Cambrian explosion and that the Burgess Shale's "weird wonders" showed that the Early Cambrian was a uniquely experimental period of animal evolution.[137] Later discoveries of similar animals and the development of new theoretical approaches led to the conclusion that many of the "weird wonders" were evolutionary "aunts" or "cousins" of modern groups[138]—for example that Opabinia was a member of the lobopods, a group which includes the ancestors of the arthropods, and that it may have been closely related to the modern tardigrades.[139] Nevertheless, there is still much debate about whether the Cambrian explosion was really explosive and, if so, how and why it happened and why it appears unique in the history of animals.[140]
Deuterostomes and the first vertebrates

Most of the animals at the heart of the Cambrian explosion debate are protostomes, one of the two main groups of complex animals. The other major group, the deuterostomes, contains invertebrates such as starfish and sea urchins (echinoderms), as well as chordates (see below). Many echinoderms have hard calcite "shells," which are fairly common from the Early Cambrian small shelly fauna onwards.[135] Other deuterostome groups are soft-bodied, and most of the significant Cambrian deuterostome fossils come from the Chengjiang fauna, a lagerstätte in China.[142] The chordates are another major deuterostome group: animals with a distinct dorsal nerve cord. Chordates include soft-bodied invertebrates such as tunicates as well as vertebrates—animals with a backbone. While tunicate fossils predate the Cambrian explosion,[143] the Chengjiang fossils Haikouichthys and Myllokunmingia appear to be true vertebrates,[18] and Haikouichthys had distinct vertebrae, which may have been slightly mineralized.[144] Vertebrates with jaws, such as the acanthodians, first appeared in the Late Ordovician.[145]
Colonization of land
Adaptation to life on land is a major challenge: all land organisms need to avoid drying-out and all those above microscopic size must create special structures to withstand gravity; respiration and gas exchange systems have to change; reproductive systems cannot depend on water to carry eggs and sperm towards each other.[146][147] Although the earliest good evidence of land plants and animals dates back to the Ordovician period (488 to 444 Ma), and a number of microorganism lineages made it onto land much earlier,[148] modern land ecosystems only appeared in the Late Devonian, about 385 to 359 Ma.[149]
Evolution of terrestrial antioxidants
Oxygen is a potent oxidant whose accumulation in terrestrial atmosphere resulted from the development of photosynthesis over 3 Ga, in cyanobacteria (blue-green algae), which were the most primitive oxygenic photosynthetic organisms. Brown algae accumulate inorganic mineral antioxidants such as rubidium, vanadium, zinc, iron, copper, molybdenum, selenium and iodine which is concentrated more than 30,000 times the concentration of this element in seawater. Protective endogenous antioxidant enzymes and exogenous dietary antioxidants helped to prevent oxidative damage. Most marine mineral antioxidants act in the cells as essential trace elements in redox and antioxidant metalloenzymes.
When plants and animals began to transfer from the sea to rivers and land about 500 Ma, environmental deficiency of these marine mineral antioxidants and iodine, was a challenge to the evolution of terrestrial life.[150][151] Terrestrial plants slowly optimized the production of “new” endogenous antioxidants such as ascorbic acid, polyphenols, flavonoids, tocopherols, etc. A few of these appeared more recently, in last 200-50 Ma, in fruits and flowers of angiosperm plants.
In fact, angiosperms (the dominant type of plant today) and most of their antioxidant pigments evolved during the Late Jurassic period. Plants employ antioxidants to defend their structures against reactive oxygen species produced during photosynthesis. Animals are exposed to the same oxidants, and they have evolved endogenous enzymatic antioxidant systems.[152] Iodine is the most primitive and abundant electron-rich essential element in the diet of marine and terrestrial organisms, and as iodide acts as an electron donor and has this ancestral antioxidant function in all iodide-concentrating cells from primitive marine algae to more recent terrestrial vertebrates.[153]
Evolution of soil
Before the colonization of land, soil, a combination of mineral particles and decomposed organic matter, did not exist. Land surfaces would have been either bare rock or unstable sand produced by weathering. Water and any nutrients in it would have drained away very quickly.[149]
Films of cyanobacteria, which are not plants but use the same photosynthesis mechanisms, have been found in modern deserts, and only in areas that are unsuitable for vascular plants. This suggests that microbial mats may have been the first organisms to colonize dry land, possibly in the Precambrian. Mat-forming cyanobacteria could have gradually evolved resistance to desiccation as they spread from the seas to intertidal zones and then to land.[149] Lichens, which are symbiotic combinations of a fungus (almost always an ascomycete) and one or more photosynthesizers (green algae or cyanobacteria),[154] are also important colonizers of lifeless environments,[149] and their ability to break down rocks contributes to soil formation in situations where plants cannot survive.[154] The earliest known ascomycete fossils date from 423 to 419 Ma in the Silurian.[149]
Soil formation would have been very slow until the appearance of burrowing animals, which mix the mineral and organic components of soil and whose feces are a major source of the organic components.[149] Burrows have been found in Ordovician sediments, and are attributed to annelids ("worms") or arthropods.[149][155]
Plants and the Late Devonian wood crisis


In aquatic algae, almost all cells are capable of photosynthesis and are nearly independent. Life on land required plants to become internally more complex and specialized: photosynthesis was most efficient at the top; roots were required in order to extract water from the ground; the parts in between became supports and transport systems for water and nutrients.[146][156]
Spores of land plants, possibly rather like liverworts, have been found in Middle Ordovician rocks dated to about 476 Ma. In Middle Silurian rocks 430 Ma, there are fossils of actual plants including clubmosses such as Baragwanathia; most were under 10 centimetres (3.9 in) high, and some appear closely related to vascular plants, the group that includes trees.[156]
By the Late Devonian 370 Ma, trees such as Archaeopteris were so abundant that they changed river systems from mostly braided to mostly meandering, because their roots bound the soil firmly.[157] In fact, they caused the "Late Devonian wood crisis"[158] because:
- They removed more carbon dioxide from the atmosphere, reducing the greenhouse effect and thus causing an ice age in the Carboniferous period.[16] In later ecosystems the carbon dioxide "locked up" in wood is returned to the atmosphere by decomposition of dead wood. However, the earliest fossil evidence of fungi that can decompose wood also comes from the Late Devonian.[159]
- The increasing depth of plants' roots led to more washing of nutrients into rivers and seas by rain. This caused algal blooms whose high consumption of oxygen caused anoxic events in deeper waters, increasing the extinction rate among deep-water animals.[16]
Land invertebrates
Animals had to change their feeding and excretory systems, and most land animals developed internal fertilization of their eggs. The difference in refractive index between water and air required changes in their eyes. On the other hand, in some ways movement and breathing became easier, and the better transmission of high-frequency sounds in air encouraged the development of hearing.[147]
The oldest known air-breathing animal is Pneumodesmus, an archipolypodan millipede from the Middle Silurian, about 428 Ma.[160][161] Its air-breathing, terrestrial nature is evidenced by the presence of spiracles, the openings to tracheal systems.[162] However, some earlier trace fossils from the Cambrian-Ordovician boundary about 490 Ma are interpreted as the tracks of large amphibious arthropods on coastal sand dunes, and may have been made by euthycarcinoids,[163] which are thought to be evolutionary "aunts" of myriapods.[164] Other trace fossils from the Late Ordovician a little over 445 Ma probably represent land invertebrates, and there is clear evidence of numerous arthropods on coasts and alluvial plains shortly before the Silurian-Devonian boundary, about 415 Ma, including signs that some arthropods ate plants.[165] Arthropods were well pre-adapted to colonise land, because their existing jointed exoskeletons provided protection against desiccation, support against gravity and a means of locomotion that was not dependent on water.[166]
The fossil record of other major invertebrate groups on land is poor: none at all for non-parasitic flatworms, nematodes or nemerteans; some parasitic nematodes have been fossilized in amber; annelid worm fossils are known from the Carboniferous, but they may still have been aquatic animals; the earliest fossils of gastropods on land date from the Late Carboniferous, and this group may have had to wait until leaf litter became abundant enough to provide the moist conditions they need.[147]
The earliest confirmed fossils of flying insects date from the Late Carboniferous, but it is thought that insects developed the ability to fly in the Early Carboniferous or even Late Devonian. This gave them a wider range of ecological niches for feeding and breeding, and a means of escape from predators and from unfavorable changes in the environment.[167] About 99% of modern insect species fly or are descendants of flying species[168]
Early land vertebrates

| "Fish" |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| |
Tetrapods, vertebrates with four limbs, evolved from other rhipidistian fish over a relatively short timespan during the Late Devonian (370 to 360 Ma).[171] The early groups are grouped together as Labyrinthodontia. They retained aquatic, fry-like tadpoles, a system still seen in modern amphibians. From the 1950s to the early 1980s it was thought that tetrapods evolved from fish that had already acquired the ability to crawl on land, possibly in order to go from a pool that was drying out to one that was deeper. However, in 1987, nearly complete fossils of Acanthostega from about 363 Ma showed that this Late Devonian transitional animal had legs and both lungs and gills, but could never have survived on land: its limbs and its wrist and ankle joints were too weak to bear its weight; its ribs were too short to prevent its lungs from being squeezed flat by its weight; its fish-like tail fin would have been damaged by dragging on the ground. The current hypothesis is that Acanthostega, which was about 1 metre (3.3 ft) long, was a wholly aquatic predator that hunted in shallow water. Its skeleton differed from that of most fish, in ways that enabled it to raise its head to breathe air while its body remained submerged, including: its jaws show modifications that would have enabled it to gulp air; the bones at the back of its skull are locked together, providing strong attachment points for muscles that raised its head; the head is not joined to the shoulder girdle and it has a distinct neck.[169]
The Devonian proliferation of land plants may help to explain why air breathing would have been an advantage: leaves falling into streams and rivers would have encouraged the growth of aquatic vegetation; this would have attracted grazing invertebrates and small fish that preyed on them; they would have been attractive prey but the environment was unsuitable for the big marine predatory fish; air-breathing would have been necessary because these waters would have been short of oxygen, since warm water holds less dissolved oxygen than cooler marine water and since the decomposition of vegetation would have used some of the oxygen.[169]
Later discoveries revealed earlier transitional forms between Acanthostega and completely fish-like animals.[172] Unfortunately, there is then a gap (Romer's gap) of about 30 Ma between the fossils of ancestral tetrapods and Middle Carboniferous fossils of vertebrates that look well-adapted for life on land. Some of these look like early relatives of modern amphibians, most of which need to keep their skins moist and to lay their eggs in water, while others are accepted as early relatives of the amniotes, whose waterproof skin enables them to live and breed far from water.[170]
Dinosaurs, birds and mammals
| Amniotes |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
Possible family tree of dinosaurs, birds and mammals[174][175]
Amniotes, whose eggs can survive in dry environments, probably evolved in the Late Carboniferous period (330 to 298.9 Ma). The earliest fossils of the two surviving amniote groups, synapsids and sauropsids, date from around 313 Ma.[174][175] The synapsid pelycosaurs and their descendants the therapsids are the most common land vertebrates in the best-known Permian (298.9 to 252.17 Ma) fossil beds. However, at the time these were all in temperate zones at middle latitudes, and there is evidence that hotter, drier environments nearer the Equator were dominated by sauropsids and amphibians.[176]
The Permian–Triassic extinction event wiped out almost all land vertebrates,[177] as well as the great majority of other life.[178] During the slow recovery from this catastrophe, estimated to have taken 30 million years,[179] a previously obscure sauropsid group became the most abundant and diverse terrestrial vertebrates: a few fossils of archosauriformes ("ruling lizard forms") have been found in Late Permian rocks,[180] but, by the Middle Triassic, archosaurs were the dominant land vertebrates. Dinosaurs distinguished themselves from other archosaurs in the Late Triassic, and became the dominant land vertebrates of the Jurassic and Cretaceous periods (201.3 to 66 Ma).[181]
During the Late Jurassic, birds evolved from small, predatory theropod dinosaurs.[182] The first birds inherited teeth and long, bony tails from their dinosaur ancestors,[182] but some had developed horny, toothless beaks by the very Late Jurassic[183] and short pygostyle tails by the Early Cretaceous.[184]
While the archosaurs and dinosaurs were becoming more dominant in the Triassic, the mammaliaform successors of the therapsids evolved into small, mainly nocturnal insectivores. This ecological role may have promoted the evolution of mammals, for example nocturnal life may have accelerated the development of endothermy ("warm-bloodedness") and hair or fur.[185] By 195 Ma in the Early Jurassic there were animals that were very like today's mammals in a number of respects.[186] Unfortunately, there is a gap in the fossil record throughout the Middle Jurassic.[187] However, fossil teeth discovered in Madagascar indicate that the split between the lineage leading to monotremes and the one leading to other living mammals had occurred by 167 Ma.[188] After dominating land vertebrate niches for about 150 Ma, the dinosaurs perished in the Cretaceous–Paleogene extinction event (66 Ma) along with many other groups of organisms.[189] Mammals throughout the time of the dinosaurs had been restricted to a narrow range of taxa, sizes and shapes, but increased rapidly in size and diversity after the extinction,[190][191] with bats taking to the air within 13 million years,[192] and cetaceans to the sea within 15 million years.[193]
Flowering plants
One possible family tree of flowering plants[194] |
Another possible family tree[195] |
The first flowering plants appeared around 130 Ma.[196] The 250,000 to 400,000 species of flowering plants outnumber all other ground plants combined, and are the dominant vegetation in most terrestrial ecosystems. There is fossil evidence that flowering plants diversified rapidly in the Early Cretaceous, from 130 to 90 Ma,[194][195] and that their rise was associated with that of pollinating insects.[195] Among modern flowering plants Magnolia are thought to be close to the common ancestor of the group.[194] However, paleontologists have not succeeded in identifying the earliest stages in the evolution of flowering plants.[194][195]
Social insects

The social insects are remarkable because the great majority of individuals in each colony are sterile. This appears contrary to basic concepts of evolution such as natural selection and the selfish gene. In fact, there are very few eusocial insect species: only 15 out of approximately 2,600 living families of insects contain eusocial species, and it seems that eusociality has evolved independently only 12 times among arthropods, although some eusocial lineages have diversified into several families. Nevertheless, social insects have been spectacularly successful; for example although ants and termites account for only about 2% of known insect species, they form over 50% of the total mass of insects. Their ability to control a territory appears to be the foundation of their success.[197]
The sacrifice of breeding opportunities by most individuals has long been explained as a consequence of these species' unusual haplodiploid method of sex determination, which has the paradoxical consequence that two sterile worker daughters of the same queen share more genes with each other than they would with their offspring if they could breed.[198] However, E. O. Wilson and Bert Hölldobler argue that this explanation is faulty: for example, it is based on kin selection, but there is no evidence of nepotism in colonies that have multiple queens. Instead, they write, eusociality evolves only in species that are under strong pressure from predators and competitors, but in environments where it is possible to build "fortresses"; after colonies have established this security, they gain other advantages through co-operative foraging. In support of this explanation they cite the appearance of eusociality in bathyergid mole rats,[197] which are not haplodiploid.[199]
The earliest fossils of insects have been found in Early Devonian rocks from about 400 Ma, which preserve only a few varieties of flightless insect. The Mazon Creek lagerstätten from the Late Carboniferous, about 300 Ma, include about 200 species, some gigantic by modern standards, and indicate that insects had occupied their main modern ecological niches as herbivores, detritivores and insectivores. Social termites and ants first appear in the Early Cretaceous, and advanced social bees have been found in Late Cretaceous rocks but did not become abundant until the Middle Cenozoic.[200]
Humans
The idea that, along with other life forms, modern-day humans evolved from an ancient, common ancestor was proposed by Robert Chambers in 1844 and taken up by Charles Darwin in 1871.[201] Modern humans evolved from a lineage of upright-walking apes that has been traced back over 6 Ma to Sahelanthropus.[202] The first known stone tools were made about 2.5 Ma, apparently by Australopithecus garhi, and were found near animal bones that bear scratches made by these tools.[203] The earliest hominines had chimpanzee-sized brains, but there has been a fourfold increase in the last 3 Ma; a statistical analysis suggests that hominine brain sizes depend almost completely on the date of the fossils, while the species to which they are assigned has only slight influence.[204] There is a long-running debate about whether modern humans evolved all over the world simultaneously from existing advanced hominines or are descendants of a single small population in Africa, which then migrated all over the world less than 200,000 years ago and replaced previous hominine species.[205] There is also debate about whether anatomically modern humans had an intellectual, cultural and technological "Great Leap Forward" under 100,000 years ago and, if so, whether this was due to neurological changes that are not visible in fossils.[206]
Mass extinctions
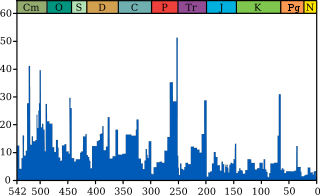
Life on Earth has suffered occasional mass extinctions at least since 542 Ma. Although they were disasters at the time, mass extinctions have sometimes accelerated the evolution of life on Earth. When dominance of particular ecological niches passes from one group of organisms to another, it is rarely because the new dominant group is "superior" to the old and usually because an extinction event eliminates the old dominant group and makes way for the new one.[25][207]
The fossil record appears to show that the gaps between mass extinctions are becoming longer and the average and background rates of extinction are decreasing. Both of these phenomena could be explained in one or more ways:[208]
- The oceans may have become more hospitable to life over the last 500 Ma and less vulnerable to mass extinctions: dissolved oxygen became more widespread and penetrated to greater depths; the development of life on land reduced the run-off of nutrients and hence the risk of eutrophication and anoxic events; and marine ecosystems became more diversified so that food chains were less likely to be disrupted.[209][210]
- Reasonably complete fossils are very rare, most extinct organisms are represented only by partial fossils, and complete fossils are rarest in the oldest rocks. So paleontologists have mistakenly assigned parts of the same organism to different genera, which were often defined solely to accommodate these finds—the story of Anomalocaris is an example of this. The risk of this mistake is higher for older fossils because these are often unlike parts of any living organism. Many of the "superfluous" genera are represented by fragments which are not found again and the "superfluous" genera appear to become extinct very quickly.[208]
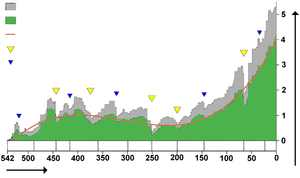
Biodiversity in the fossil record, which is "...the number of distinct genera alive at any given time; that is, those whose first occurrence predates and whose last occurrence postdates that time"[211] shows a different trend: a fairly swift rise from 542 to 400 Ma; a slight decline from 400 to 200 Ma, in which the devastating Permian–Triassic extinction event is an important factor; and a swift rise from 200 Ma to the present.[211]
See also
- Constructal law
- Evolution
- Evolution of mammals
- Evolution of plants
- Evolution of sexual reproduction
- History of evolutionary thought
- On the Origin of Species
- Speciation
- Taxonomy of commonly fossilised invertebrates
- Timeline of evolutionary history of life
- Treatise on Invertebrate Paleontology
- Viral evolution
Footnotes
- ↑ Name given as in Nicholas J. Butterfield's paper published by Paleobiology, "Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes" (Summer 2000). A fossil fish, also from China, has also been named Qingshania. The name of one of these will have to change.
- ↑ Myxozoa were thought to be an exception, but are now thought to be heavily modified members of the Cnidaria. Jímenez-Guri, Eva; Philippe, Hervé; Okamura, Beth; Holland, Peter W. H. (July 6, 2007). "Buddenbrockia Is a Cnidarian Worm". Science (Washington, D.C.: American Association for the Advancement of Science) 317 (5834): 116–118. Bibcode:2007Sci...317..116J. doi:10.1126/science.1142024. ISSN 0036-8075. PMID 17615357. Retrieved 2008-09-03.
References
- ↑ Futuyma 2005
- ↑ Stearns & Stearns 1999, p. x
- ↑ Novacek, Michael J. (November 8, 2014). "Prehistory’s Brilliant Future". The New York Times (New York: The New York Times Company). ISSN 0362-4331. Retrieved 2014-12-25.
- ↑ Miller & Spoolman 2012, p. 62
- ↑ Ohtomo, Yoko; Kakegawa, Takeshi; Ishida, Akizumi et al. (2014). "Evidence for biogenic graphite in early Archaean Isua metasedimentary rocks". Nature Geoscience (London: Nature Publishing Group) 7: 25–28. doi:10.1038/ngeo2025. ISSN 1752-0894. Retrieved 2015-01-22.
- ↑ Borenstein, Seth (November 13, 2013). "Oldest fossil found: Meet your microbial mom". Excite News (New York: IAC/InterActiveCorp). Associated Press. Retrieved 2013-11-15.
- ↑ Noffke, Nora; Christian, Daniel; Wacey, David; Hazen, Robert M. (December 16, 2013). "Microbially Induced Sedimentary Structures Recording an Ancient Ecosystem in the ca. 3.48 Billion-Year-Old Dresser Formation, Pilbara, Western Australia". Astrobiology (New Rochelle, NY: Mary Ann Liebert, Inc.) 13 (12): 1103–1124. Bibcode:2013AsBio..13.1103N. doi:10.1089/ast.2013.1030. ISSN 1531-1074. PMC 3870916. PMID 24205812. Retrieved 2013-11-15.
- ↑ 8.0 8.1 8.2 Nisbet, Euan G.; Fowler, C. M. R. (December 7, 1999). "Archaean metabolic evolution of microbial mats". Proceedings of the Royal Society B (London: Royal Society) 266 (1436): 2375–2382. doi:10.1098/rspb.1999.0934. ISSN 0962-8452. PMC 1690475.
- ↑ Anbar, A.; Duan, Y.; Lyons, T.; Arnold, G.; Kendall, B.; Creaser, R.; Kaufman, A.; Gordon, G.; Scott, C.; Garvin, J.; Buick, R. (2007). "A whiff of oxygen before the great oxidation event?". Science 317 (5846): 1903–1906. Bibcode:2007Sci...317.1903A. doi:10.1126/science.1140325. PMID 17901330.
- ↑ Knoll, Andrew H.; Javaux, Emmanuelle J.; Hewitt, David; Cohen, Phoebe (June 29, 2006). "Eukaryotic organisms in Proterozoic oceans". Philosophical Transactions of the Royal Society B (London: Royal Society) 361 (1470): 1023–1038. doi:10.1098/rstb.2006.1843. ISSN 0962-8436. PMC 1578724. PMID 16754612.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 Fedonkin, Mikhail A. (March 31, 2003). "The origin of the Metazoa in the light of the Proterozoic fossil record" (PDF). Paleontological Research (Tokyo: Palaeontological Society of Japan) 7 (1): 9–41. doi:10.2517/prpsj.7.9. ISSN 1880-0068. Retrieved 2008-09-02.
- ↑ 12.0 12.1 12.2 Bonner, John Tyler (1998). "The origins of multicellularity". Integrative Biology (London: Royal Society of Chemistry) 1 (1): 27–36. doi:10.1002/(SICI)1520-6602(1998)1:1<27::AID-INBI4>3.0.CO;2-6. ISSN 1757-9694. Retrieved 2008-09-03.
- ↑ Fedonkin, Mikhail A.; Simonetta, Alberto; Ivantsov, Andrei Yu. (2007). "New data on Kimberella, the Vendian mollusc-like organism (White Sea region, Russia): palaeoecological and evolutionary implications" (PDF). Geological Society Special Publication (Oxford; Boston: Published for the Geological Society of London by Blackwell Scientific Publications) 286: 157–179. Bibcode:2007GSLSP.286..157F. doi:10.1144/SP286.12. ISSN 0305-8719. Retrieved 2013-05-16.
- ↑ Ciesielski, Paul F. "Transition of Plants to Land". Gainesville, FL: University of Florida. Archived from the original on 2013-11-02. Retrieved 2015-01-22.
The oldest fossils reveal evolution of non-vascular plants by the middle to late Ordovician Period (~450-440 m.y.a.) on the basis of fossil spores.
- ↑ Beraldi-Campesi, Hugo (February 2013). "Early life on land and the first terrestrial ecosystems". Ecological Processes (Heidelberg: Springer-Verlag) 2 (1). doi:10.1186/2192-1709-2-1. ISSN 2192-1709. Retrieved 2015-01-21.
- ↑ 16.0 16.1 16.2 Algeo, Thomas J.; Scheckler, Stephen E. (January 29, 1998). "Terrestrial-marine teleconnections in the Devonian: links between the evolution of land plants, weathering processes, and marine anoxic events". Philosophical Transactions of the Royal Society B (London: Royal Society) 353 (1365): 113–130. doi:10.1098/rstb.1998.0195. ISSN 0962-8436. PMC 1692181.
- ↑ Jun-Yuan Chen; Oliveri, Paola; Chia-Wei Li et al. (April 25, 2000). "Precambrian animal diversity: Putative phosphatized embryos from the Doushantuo Formation of China". Proc. Natl. Acad. Sci. U.S.A. (Washington, D.C.: National Academy of Sciences) 97 (9): 4457–4462. Bibcode:2000PNAS...97.4457C. doi:10.1073/pnas.97.9.4457. ISSN 0027-8424. PMC 18256. PMID 10781044. Retrieved 2009-04-30.
- ↑ 18.0 18.1 D-G. Shu; H-L. Luo; Conway Morris, Simon et al. (November 4, 1999). "Lower Cambrian vertebrates from south China" (PDF). Nature (London: Nature Publishing Group) 402 (6757): 42–46. Bibcode:1999Natur.402...42S. doi:10.1038/46965. ISSN 0028-0836. Archived from the original on 2009-02-26. Retrieved 2015-01-22.
- ↑ Hoyt, Donald F. (February 17, 1997). "Synapsid Reptiles". ZOO 138 Vertebrate Zoology (Lecture). Pomona, CA: California State Polytechnic University, Pomona. Archived from the original on 2009-05-20. Retrieved 2015-01-22.
- ↑ Barry, Patrick L. (January 28, 2002). Phillips, Tony, ed. "The Great Dying". Science@NASA. Science and Technology Directorate, Marshall Space Flight Center. Retrieved 2015-01-22.
- ↑ Tanner, Lawrence H.; Lucas, Spencer G.; Chapman, Mary G. (March 2004). "Assessing the record and causes of Late Triassic extinctions" (PDF). Earth-Science Reviews (Amsterdam, the Netherlands: Elsevier) 65 (1–2): 103–139. Bibcode:2004ESRv...65..103T. doi:10.1016/S0012-8252(03)00082-5. ISSN 0012-8252. Archived from the original on 2007-10-25. Retrieved 2007-10-22.
- ↑ Benton 1997
- ↑ Fastovsky, David E.; Sheehan, Peter M. (March 2005). "The Extinction of the Dinosaurs in North America" (PDF). GSA Today (Boulder, CO: Geological Society of America) 15 (3): 4–10. doi:10.1130/1052-5173(2005)015<4:TEOTDI>2.0.CO;2. ISSN 1052-5173. Retrieved 2015-01-23.
- ↑ Roach, John (June 20, 2007). "Dinosaur Extinction Spurred Rise of Modern Mammals". National Geographic News (Washington, D.C.: National Geographic Society). Retrieved 2009-03-08.
- ↑ 25.0 25.1 Van Valkenburgh, Blaire (May 1999). "Major patterns in the history of carnivorous mammals". Annual Review of Earth and Planetary Sciences (Palo Alto, CA: Annual Reviews) 27: 463–493. Bibcode:1999AREPS..27..463V. doi:10.1146/annurev.earth.27.1.463. ISSN 1545-4495. Retrieved 2015-01-23.
- ↑ 26.0 26.1 El Albani, Abderrazak; Bengtson, Stefan; Canfield, Donald E. et al. (July 1, 2010). "Large colonial organisms with coordinated growth in oxygenated environments 2.1 Gyr ago". Nature (London: Nature Publishing Group) 466 (7302): 100–104. Bibcode:2010Natur.466..100A. doi:10.1038/nature09166. ISSN 0028-0836. PMID 20596019.
- ↑ Dalrymple 1991
- Newman 2007
- Dalrymple, G. Brent (2001). "The age of the Earth in the twentieth century: a problem (mostly) solved". Geological Society Special Publication (Oxford; Boston: Published for the Geological Society of London by Blackwell Scientific Publications) 190: 205–221. Bibcode:2001GSLSP.190..205D. doi:10.1144/GSL.SP.2001.190.01.14. ISSN 0305-8719. Retrieved 2015-01-23.
- ↑ Galimov, Erik M.; Krivtsov, Anton M. (December 2005). "Origin of the Earth—Moon system" (PDF). Journal of Earth System Science (Springer Science+Business Media on behalf of the Indian Academy of Sciences) 114 (6): 593–600. Bibcode:2005JESS..114..593G. doi:10.1007/BF02715942. ISSN 0253-4126. Retrieved 2015-01-23.
- ↑ Thompson, Andrea (September 25, 2008). "Oldest Rocks on Earth Found". LiveScience (Watsonville, CA: Imaginova). Retrieved 2015-01-23.
- ↑ Dalrymple 1991
- ↑ Newman 2007
- ↑ Dalrymple, G. Brent (2001). "The age of the Earth in the twentieth century: a problem (mostly) solved". Geological Society Special Publication (Oxford; Boston: Published for the Geological Society of London by Blackwell Scientific Publications) 190: 205–221. Bibcode:2001GSLSP.190..205D. doi:10.1144/GSL.SP.2001.190.01.14. ISSN 0305-8719. Retrieved 2015-01-23.
- ↑ 33.0 33.1 Cohen, Barbara A.; Swindle, Timothy D.; Kring, David A. (December 1, 2000). "Support for the Lunar Cataclysm Hypothesis from Lunar Meteorite Impact Melt Ages". Science (Washington, D.C.: American Association for the Advancement of Science) 290 (5497): 1754–1756. Bibcode:2000Sci...290.1754C. doi:10.1126/science.290.5497.1754. ISSN 0036-8075. PMID 11099411. Retrieved 2015-01-23.
- ↑ "Early Earth Likely Had Continents And Was Habitable" (Press release). Boulder, CO: University of Colorado. November 17, 2005. Retrieved 2015-01-23.
- ↑ Cavosie, Aaron J.; Valley, John W.; Wilde, Simon A.; Edinburgh Ion Microprobe Facility (July 15, 2005). "Magmatic δ18O in 4400-3900 Ma detrital zircons: A record of the alteration and recycling of crust in the Early Archean". Earth and Planetary Science Letters (Amsterdam, the Netherlands: Elsevier) 235 (3–4): 663–681. Bibcode:2005E&PSL.235..663C. doi:10.1016/j.epsl.2005.04.028. ISSN 0012-821X.
- ↑ Britt, Robert Roy (July 24, 2002). "Evidence for Ancient Bombardment of Earth". Space.com (New York: Space Holding Corp.). Archived from the original on 2006-04-15. Retrieved 2015-01-23.
- ↑ Valley, John W.; Peck, William H.; King, Elizabeth M.; Wilde, Simon A. (April 2002). "A cool early Earth" (PDF). Geology (Boulder, CO: Geological Society of America) 30 (4): 351–354. Bibcode:2002Geo....30..351V. doi:10.1130/0091-7613(2002)030<0351:ACEE>2.0.CO;2. ISSN 0091-7613. Retrieved 2008-09-13.
- ↑ Dauphas, Nicolas; Robert, François; Marty, Bernard (December 2000). "The Late Asteroidal and Cometary Bombardment of Earth as Recorded in Water Deuterium to Protium Ratio". Icarus (Amsterdam, the Netherlands: Elsevier) 148 (2): 508–512. Bibcode:2000Icar..148..508D. doi:10.1006/icar.2000.6489. ISSN 0019-1035.
- ↑ Scalice, Daniella (May 20, 2009). Fletcher, Julie, ed. "Microbial Habitability During the Late Heavy Bombardment". Astrobiology. Mountain View, CA: NASA Astrobiology Program. Retrieved 2015-01-23.
- ↑ 40.0 40.1 Brasier, Martin; McLoughlin, Nicola; Green, Owen; Wacey, David (June 2006). "A fresh look at the fossil evidence for early Archaean cellular life" (PDF). Philosophical Transactions of the Royal Society B (London: Royal Society) 361 (1470): 887–902. doi:10.1098/rstb.2006.1835. ISSN 0962-8436. PMC 1578727. PMID 16754605. Retrieved 2008-08-30.
- ↑ Schopf, J. William (April 30, 1993). "Microfossils of the Early Archean Apex Chert: New Evidence of the Antiquity of Life". Science (Washington, D.C.: American Association for the Advancement of Science) 260 (5108): 640–646. Bibcode:1993Sci...260..640S. doi:10.1126/science.260.5108.640. ISSN 0036-8075. PMID 11539831. Retrieved 2015-01-24.
- Altermann, Wladyslaw; Kazmierczak, Józef (November 2003). "Archean microfossils: a reappraisal of early life on Earth". Research in Microbiology (Amsterdam, the Netherlands: Elsevier for the Pasteur Institute) 154 (9): 611–617. doi:10.1016/j.resmic.2003.08.006. ISSN 0923-2508. PMID 14596897.
- ↑ Mojzsis, Stephen J.; Arrhenius, Gustaf; McKeegan, Kevin D. et al. (November 1996). "Evidence for life on Earth before 3,800 million years ago". Nature (London: Nature Publishing Group) 384 (6604): 55–59. Bibcode:1996Natur.384...55M. doi:10.1038/384055a0. ISSN 0028-0836. PMID 8900275. Retrieved 2008-08-30.
- ↑ 43.0 43.1 Grotzinger, John P.; Rothman, Daniel H. (October 3, 1996). "An abiotic model for stromatolite morphogenesis". Nature (London: Nature Publishing Group) 383 (6599): 423–425. Bibcode:1996Natur.383..423G. doi:10.1038/383423a0. ISSN 0028-0836.
- ↑ Fedo, Christopher M.; Whitehouse, Martin J. (May 24, 2002). "Metasomatic Origin of Quartz-Pyroxene Rock, Akilia, Greenland, and Implications for Earth's Earliest Life". Science (Washington, D.C.: American Association for the Advancement of Science) 296 (5572): 1448–1452. Bibcode:2002Sci...296.1448F. doi:10.1126/science.1070336. ISSN 0036-8075. PMID 12029129. Retrieved 2015-01-24.
- Lepland, Aivo; van Zuilen, Mark A.; Arrhenius, Gustaf et al. (January 2005). "Questioning the evidence for Earth's earliest life—Akilia revisited". Geology (Boulder, CO: Geological Society of America) 33 (1): 77–79. Bibcode:2005Geo....33...77L. doi:10.1130/G20890.1. ISSN 0091-7613. Retrieved 2015-01-24.
- ↑ Schopf, J. William (June 29, 2006). "Fossil evidence of Archaean life". Philosophical Transactions of the Royal Society B (London: Royal Society) 361 (1470): 869–885. doi:10.1098/rstb.2006.1834. ISSN 0962-8436. PMC 1578735. PMID 16754604.
- ↑ Mason, Stephen F. (1984). "Origins of biomolecular handedness". Nature (London: Nature Publishing Group) 311 (5981): 19–23. Bibcode:1984Natur.311...19M. doi:10.1038/311019a0. ISSN 0028-0836. PMID 6472461.
- ↑ Orgel, Leslie E. (October 1994). "The Origin of Life on the Earth" (PDF). Scientific American (Stuttgart: Georg von Holtzbrinck Publishing Group) 271 (4): 76–83. doi:10.1038/scientificamerican1094-76. ISSN 0036-8733. PMID 7524147. Retrieved 2008-08-30. "The Origin of Life on the Earth" at the Wayback Machine (archived 6 November 2008).
- ↑ O'Leary 2008
- ↑ 49.0 49.1 Arrhenius 1980, p. 32
- ↑ Hoyle, Fred; Wickramasinghe, Nalin C. (November 1979). "On the Nature of Interstellar Grains". Astrophysics and Space Science (Dordrecht, the Netherlands: Kluwer Academic Publishers) 66 (1): 77–90. Bibcode:1979Ap&SS..66...77H. doi:10.1007/BF00648361. ISSN 0004-640X.
- ↑ 51.0 51.1 Crick, Francis H.; Orgel, Leslie E (July 1973). "Directed Panspermia". Icarus (Amsterdam, the Netherlands: Elsevier) 19 (3): 341–348. Bibcode:1973Icar...19..341C. doi:10.1016/0019-1035(73)90110-3. ISSN 0019-1035.
- ↑ 52.0 52.1 52.2 Warmflash, David; Weiss, Benjamin (November 2005). "Did Life Come From Another World?". Scientific American (Stuttgart: Georg von Holtzbrinck Publishing Group) 293 (5): 64–71. doi:10.1038/scientificamerican1105-64. ISSN 0036-8733. Retrieved 2015-01-25.
- ↑ Wickramasinghe, Nalin C.; Wickramasinghe, Janaki T. (September 2008). "On the possibility of microbiota transfer from Venus to Earth". Astrophysics and Space Science (Dordrecht, the Netherlands: Springer Netherlands) 317 (1–2): 133–137. Bibcode:2008Ap&SS.317..133W. doi:10.1007/s10509-008-9851-2. ISSN 0004-640X.
- ↑ Clancy, Brack & Horneck 2005
- ↑ Horneck, Gerda; Klaus, David M.; Mancinelli, Rocco L. (March 2010). "Space Microbiology". Microbiology and Molecular Biology Reviews (Washington, D.C.: American Society for Microbiology) 74 (1): 121–156. doi:10.1128/mmbr.00016-09. ISSN 1092-2172. Retrieved 2013-07-29.
- ↑ Than, Ker (August 23, 2007). "Claim of Martian Life Called 'Bogus'". Space.com (Watsonville, CA: Imaginova). Retrieved 2015-01-25.
- ↑ Bennett 2008, pp. 82–85
- ↑ Schulze-Makuch, Dirk; Irwin, Louis N. (April 2006). "The prospect of alien life in exotic forms on other worlds". Naturwissenschaften (Heidelberg: Springer Science+Business Media) 93 (4): 155–72. Bibcode:2006NW.....93..155S. doi:10.1007/s00114-005-0078-6. ISSN 0028-1042. PMID 16525788.
- ↑ Peretó, Juli (2005). "Controversies on the origin of life" (PDF). International Microbiology (Barcelona: Spanish Society for Microbiology) 8 (1): 23–31. ISSN 1139-6709. PMID 15906258. Retrieved 2007-10-07.
- ↑ Szathmáry, Eörs (February 3, 2005). "Life: In search of the simplest cell". Nature (London: Nature Publishing Group) 433 (7025): 469–470. Bibcode:2005Natur.433..469S. doi:10.1038/433469a. ISSN 0028-0836. PMID 15690023. Retrieved 2008-09-01.
- ↑ Luisi, Pier Luigi; Ferri, Francesca; Stano, Pasquale (January 2006). "Approaches to semi-synthetic minimal cells: a review". Naturwissenschaften (Heidelberg: Springer Science+Business Media) 93 (1): 1–13. Bibcode:2006NW.....93....1L. doi:10.1007/s00114-005-0056-z. ISSN 0028-1042. PMID 16292523.
- ↑ Joyce, Gerald F. (July 11, 2002). "The antiquity of RNA-based evolution". Nature (London: Nature Publishing Group) 418 (6894): 214–221. Bibcode:2002Natur.418..214J. doi:10.1038/418214a. ISSN 0028-0836. PMID 12110897.
- ↑ 63.0 63.1 Hoenigsberg, Hugo (December 30, 2003). "Evolution without speciation but with selection: LUCA, the Last Universal Common Ancestor in Gilbert's RNA world". Genetic and Molecular Research (Ribeirão Preto, Brazil: Fundação de Pesquisas Científicas de Ribeirão Preto) 2 (4): 366–375. ISSN 1676-5680. PMID 15011140. Retrieved 2008-08-30.
- ↑ Trevors, Jack T.; Abel, David L. (November 2004). "Chance and necessity do not explain the origin of life". Cell Biology International (London: Portland Press for the International Federation for Cell Biology) 28 (11): 729–739. doi:10.1016/j.cellbi.2004.06.006. ISSN 1065-6995. PMID 15563395.
- ↑ Forterre, Patrick; Benachenhou-Lahfa, Nadia; Confalonieri, Fabrice et al. (1992). "The nature of the last universal ancestor and the root of the tree of life, still open questions". BioSystems (Amsterdam, the Netherlands: Elsevier) 28 (1–3): 15–32. doi:10.1016/0303-2647(92)90004-I. ISSN 0303-2647. PMID 1337989.
- ↑ Cech, Thomas R. (August 11, 2000). "The Ribosome Is a Ribozyme". Science (Washington, D.C.: American Association for the Advancement of Science) 289 (5481): 878–879. doi:10.1126/science.289.5481.878. ISSN 0036-8075. PMID 10960319. Retrieved 2015-01-26.
- ↑ Johnston, Wendy K.; Unrau, Peter J.; Lawrence, Michael S. et al. (May 18, 2001). "RNA-Catalyzed RNA Polymerization: Accurate and General RNA-Templated Primer Extension". Science (Washington, D.C.: American Association for the Advancement of Science) 292 (5520): 1319–1325. Bibcode:2001Sci...292.1319J. doi:10.1126/science.1060786. ISSN 0036-8075. PMID 11358999.
- ↑ 68.0 68.1 Levy, Matthew; Miller, Stanley L. (July 7, 1998). "The stability of the RNA bases: Implications for the origin of life". Proc. Natl. Acad. Sci. U.S.A. (Washington, D.C.: National Academy of Sciences) 95 (14): 7933–7938. Bibcode:1998PNAS...95.7933L. doi:10.1073/pnas.95.14.7933. ISSN 0027-8424. PMC 20907. PMID 9653118. Retrieved 2015-01-26.
- Larralde, Rosa; Robertson, Michael P.; Miller, Stanley L. (August 29, 1995). "Rates of decomposition of ribose and other sugars: Implications for chemical evolution". Proc. Natl. Acad. Sci. U.S.A. (Washington, D.C.: National Academy of Sciences) 92 (18): 8158–8160. Bibcode:1995PNAS...92.8158L. doi:10.1073/pnas.92.18.8158. ISSN 0036-8075. PMC 41115. PMID 7667262. Retrieved 2015-01-26.
- Lindahl, Tomas (April 22, 1993). "Instability and decay of the primary structure of DNA". Nature (London: Nature Publishing Group) 362 (6422): 709–715. Bibcode:1993Natur.362..709L. doi:10.1038/362709a0. ISSN 0028-0836. PMID 8469282.
- ↑ Orgel, Leslie (November 17, 2000). "A Simpler Nucleic Acid". Science (Washington, D.C.: American Association for the Advancement of Science) 290 (5495): 1306–1307. doi:10.1126/science.290.5495.1306. ISSN 0036-8075. PMID 11185405.
- ↑ Nelson, Kevin E.; Levy, Matthew; Miller, Stanley L. (April 11, 2000). "Peptide nucleic acids rather than RNA may have been the first genetic molecule". Proc. Natl. Acad. Sci. U.S.A. (Washington, D.C.) 97 (8): 3868–3871. Bibcode:2000PNAS...97.3868N. doi:10.1073/pnas.97.8.3868. ISSN 0036-8075. PMC 18108. PMID 10760258. Retrieved 2015-01-26.
- ↑ Martin, William; Russell, Michael J. (January 29, 2003). "On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells". Philosophical Transactions of the Royal Society B (London: Royal Society) 358 (1429): 59–85. doi:10.1098/rstb.2002.1183. ISSN 0962-8436. PMC 1693102. PMID 12594918.
- ↑ Wächtershäuser, Günter (August 25, 2000). "Life as We Don't Know It". Science (Washington, D.C.: American Association for the Advancement of Science) 289 (5483): 1307–1308. doi:10.1126/science.289.5483.1307. ISSN 0036-8075. PMID 10979855.
- ↑ Trevors, Jack T.; Psenner, Roland (December 2001). "From self-assembly of life to present-day bacteria: a possible role for nanocells". FEMS Microbiology Reviews (Amsterdam, the Netherlands: Elsevier on behalf of the Federation of European Microbiological Societies) 25 (5): 573–582. doi:10.1111/j.1574-6976.2001.tb00592.x. ISSN 1574-6976. PMID 11742692.
- ↑ Segré, Daniel; Ben-Eli, Dafna; Deamer, David W.; Lancet, Doron (February 2001). "The Lipid World" (PDF). Origins of Life and Evolution of Biospheres (Dordrecht, the Netherlands: Kluwer Academic Publishers) 31 (1–2): 119–145. doi:10.1023/A:1006746807104. ISSN 0169-6149. PMID 11296516. Retrieved 2015-01-26.
- ↑ Cairns-Smith 1968, pp. 57–66
- ↑ Ferris, James P. (June 1999). "Prebiotic Synthesis on Minerals: Bridging the Prebiotic and RNA Worlds". The Biological Bulletin (Woods Hole, MA: Marine Biological Laboratory) 196 (3): 311–314. doi:10.2307/1542957. ISSN 0006-3185. JSTOR 1542957. PMID 10390828. "This paper was originally presented at a workshop titled Evolution: A Molecular Point of View."
- ↑ Hanczyc, Martin M.; Fujikawa, Shelly M.; Szostak, Jack W. (October 24, 2003). "Experimental Models of Primitive Cellular Compartments: Encapsulation, Growth, and Division". Science (Washington, D.C.: American Association for the Advancement of Science) 302 (5645): 618–622. Bibcode:2003Sci...302..618H. doi:10.1126/science.1089904. ISSN 0036-8075. PMID 14576428. Retrieved 2015-01-26.
- ↑ Hartman, Hyman (October 1998). "Photosynthesis and the Origin of Life". Origins of Life and Evolution of Biospheres (Dordrecht, the Netherlands: Kluwer Academic Publishers) 28 (4–6): 512–521. Bibcode:1998OLEB...28..515H. doi:10.1023/A:1006548904157. ISSN 0169-6149. Retrieved 2015-01-27.
- ↑ 79.0 79.1 Krumbein et al. 2003, pp. 1–28
- ↑ 80.0 80.1 Risatti, J. Bruno; Capman, William C.; Stahl, David A. (October 11, 1994). "Community structure of a microbial mat: The phylogenetic dimension" (PDF). Proc. Natl. Acad. Sci. U.S.A. (Washington, D.C.: National Academy of Sciences) 91 (21): 10173–10177. Bibcode:1994PNAS...9110173R. doi:10.1073/pnas.91.21.10173. ISSN 0036-8075. PMC 44980. PMID 7937858. Retrieved 2008-07-09.
- ↑ "Biodiversity rocks". Nature (Editor's summary) (London: Nature Publishing Group) 441 (7094). June 8, 2006. ISSN 0028-0836. Retrieved 2009-01-10.
- ↑ Allwood, Abigail C.; Walter, Malcolm R.; Kamber, Balz S. et al. (June 8, 2006). "Stromatolite reef from the Early Archaean era of Australia". Nature (London: Nature Publishing Group) 441 (7094): 714–718. Bibcode:2006Natur.441..714A. doi:10.1038/nature04764. ISSN 0028-0836. PMID 16760969. Retrieved 2008-08-31.
- ↑ Blankenship, Robert E. (January 1, 2001). "Molecular evidence for the evolution of photosynthesis". Trends in Plant Science (Cambridge, MA: Cell Press) 6 (1): 4–6. doi:10.1016/S1360-1385(00)01831-8. ISSN 1360-1385. PMID 11164357. Retrieved 2015-01-28.
- ↑ Hoehler, Tori M.; Bebout, Brad M.; Des Marais, David J. (July 19, 2001). "The role of microbial mats in the production of reduced gases on the early Earth". Nature (London: Nature Publishing Group) 412 (6844): 324–327. doi:10.1038/35085554. ISSN 0028-0836. PMID 11460161. Retrieved 2008-07-14.
- ↑ Abele, Doris (November 7, 2002). "Toxic oxygen: The radical life-giver". Nature (London: Nature Publishing Group) 420 (6911): 27. Bibcode:2002Natur.420...27A. doi:10.1038/420027a. ISSN 0028-0836. PMID 12422197. Retrieved 2008-07-14.
- ↑ Westerdahl, Becky B. (2007). "Introduction to Aerobic Respiration". Biological Science 10V (Lecture). Davis, CA: University of California, Davis. Archived from the original on 2007-10-29. Retrieved 2008-07-14.
- Textbook used for lecture: Biology Today and Tomorrow With Physiology (2007), ISBN 0-495-01654-3.
- ↑ Goldblatt, Colin; Lenton, Timothy M.; Watson, Andrew J. (2006). "The Great Oxidation at ~2.4 Ga as a bistability in atmospheric oxygen due to UV shielding by ozone" (PDF). Geophysical Research Abstracts (Katlenburg-Lindau, Germany: Copernicus Publications on behalf of the European Geosciences Union) 8 (00770). ISSN 1029-7006. SRef-ID: 1607-7962/gra/EGU06-A-00770. Retrieved 2008-09-01.
- ↑ 88.0 88.1 Glansdorff, Nicolas; Ying Xu; Labedan, Bernard (July 9, 2008). "The Last Universal Common Ancestor: emergence, constitution and genetic legacy of an elusive forerunner". Biology Direct (London: BioMed Central) 3 (29): 29. doi:10.1186/1745-6150-3-29. ISSN 1745-6150. PMC 2478661. PMID 18613974.
- ↑ 89.0 89.1 Brocks, Jochen J.; Logan, Graham A.; Buick, Roger; Summons, Roger E. (August 13, 1999). "Archean Molecular Fossils and the Early Rise of Eukaryotes". Science (Washington, D.C.: American Association for the Advancement of Science) 285 (5430): 1033–1036. doi:10.1126/science.285.5430.1033. ISSN 0036-8075. PMID 10446042. Retrieved 2015-01-29.
- ↑ 90.0 90.1 90.2 Hedges, S. Blair; Blair, Jaime E.; Venturi, Maria L.; Shoe, Jason L. (January 28, 2004). "A molecular timescale of eukaryote evolution and the rise of complex multicellular life". BMC Evolutionary Biology (London: BioMed Central) 4: 2. doi:10.1186/1471-2148-4-2. ISSN 1471-2148. PMC 341452. PMID 15005799. Retrieved 2008-07-14.
- ↑ Burki, Fabien; Shalchian-Tabrizi, Kamran; Minge, Marianne et al. (August 29, 2007). Butler, Geraldine, ed. "Phylogenomics Reshuffles the Eukaryotic Supergroups". PLOS ONE (San Francisco, CA: Public Library of Science) 2 (8): e790. Bibcode:2007PLoSO...2..790B. doi:10.1371/journal.pone.0000790. ISSN 1932-6203. PMC 1949142. PMID 17726520.
- ↑ Parfrey, Laura Wegener; Barbero, Erika; Lasser, Elyse et al. (December 22, 2006). "Evaluating Support for the Current Classification of Eukaryotic Diversity". PLOS Genetics (San Francisco, CA: Public Library of Science) 2 (12): e220. doi:10.1371/journal.pgen.0020220. ISSN 1553-7404. PMC 1713255. PMID 17194223.
- ↑ Margulis 1981
- ↑ Vellai, Tibor; Vida, Gábor (August 7, 1999). "The origin of eukaryotes: the difference between prokaryotic and eukaryotic cells". Proceedings of the Royal Society B (London: Royal Society) 266 (1428): 1571–1577. doi:10.1098/rspb.1999.0817. ISSN 0962-8452. PMC 1690172. PMID 10467746.
- ↑ Selosse, Marc-André; Abert, Béatrice; Godelle, Bernard (March 1, 2001). "Reducing the genome size of organelles favours gene transfer to the nucleus". Trends in Ecology & Evolution (Cambridge, MA: Cell Press) 16 (3): 135–141. doi:10.1016/S0169-5347(00)02084-X. ISSN 0169-5347. Retrieved 2015-01-29.
- ↑ Pisani, Davide; Cotton, James A.; McInerney, James O. (August 2007). "Supertrees Disentangle the Chimerical Origin of Eukaryotic Genomes". Molecular Biology and Evolution (Oxford: Oxford University Press on behalf of the Society for Molecular Biology and Evolution) 24 (8): 1752–1760. doi:10.1093/molbev/msm095. ISSN 0737-4038. PMID 17504772.
- ↑ Gray, Michael W.; Burger, Gertraud; Lang, B. Franz (March 5, 1999). "Mitochondrial Evolution". Science (Washington, D.C.: American Association for the Advancement of Science) 283 (5407): 1476–1481. Bibcode:1999Sci...283.1476G. doi:10.1126/science.283.5407.1476. ISSN 0036-8075. PMID 10066161. Retrieved 2015-01-29.
- ↑ Rasmussen, Birger; Fletcher, Ian R.; Brocks, Jochen J.; Kilburn, Matt R. (October 23, 2008). "Reassessing the first appearance of eukaryotes and cyanobacteria". Nature (London: Nature Publishing Group) 455 (7216): 1101–1104. Bibcode:2008Natur.455.1101R. doi:10.1038/nature07381. ISSN 0028-0836. PMID 18948954.
- ↑ Tsu-Ming Han; Runnegar, Bruce (July 10, 1992). "Megascopic eukaryotic algae from the 2.1-billion-year-old negaunee iron-formation, Michigan". Science (Washington, D.C.: American Association for the Advancement of Science) 257 (5067): 232–235. Bibcode:1992Sci...257..232H. doi:10.1126/science.1631544. ISSN 0036-8075. PMID 1631544. Retrieved 2015-01-30.
- ↑ Javaux, Emmanuelle J.; Knoll, Andrew H.; Walter, Malcolm R. (July 2004). "TEM evidence for eukaryotic diversity in mid-Proterozoic oceans". Geobiology (Hoboken, NJ: Wiley-Blackwell) 2 (3): 121–132. doi:10.1111/j.1472-4677.2004.00027.x. ISSN 1472-4677. Retrieved 2015-01-30.
- ↑ 101.0 101.1 Butterfield, Nicholas J. (Winter 2005). "Probable Proterozoic fungi". Paleobiology (Paleontological Society) 31 (1): 165–182. doi:10.1666/0094-8373(2005)031<0165:PPF>2.0.CO;2. ISSN 0094-8373. Retrieved 2015-01-30.
- ↑ 102.0 102.1 102.2 102.3 102.4 Neiman, Maurine; Jokela, Jukka (2010). "Sex: Advantage". Encyclopedia of Life Sciences. Hoboken, NJ: John Wiley & Sons. doi:10.1002/9780470015902.a0001716.pub2. ISBN 0-470-01617-5. Retrieved 2015-01-21.
- ↑ Holmes & Jobling 1996
- ↑ Christie, Peter J. (April 2001). "Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines". Molecular Microbiology (Hoboken, NJ: Wiley-Blackwell) 40 (2): 294–305. doi:10.1046/j.1365-2958.2001.02302.x. ISSN 0950-382X. PMID 11309113.
- ↑ Michod, Richard E.; Bernstein, Harris; Nedelcu, Aurora M. (May 2008). "Adaptive value of sex in microbial pathogens" (PDF). Infection, Genetics and Evolution (Amsterdam, the Netherlands: Elsevier) 8 (3): 267–285. doi:10.1016/j.meegid.2008.01.002. ISSN 1567-1348. PMID 18295550.
- ↑ Bernstein, Harris; Bernstein, Carol (July 2010). "Evolutionary Origin of Recombination during Meiosis". BioScience (Oxford: Oxford University Press on behalf of the American Institute of Biological Sciences) 60 (7): 498–505. doi:10.1525/bio.2010.60.7.5. ISSN 0006-3568.
- ↑ Johnsborg, Ola; Eldholm, Vegard; Håvarstein, Leiv Sigve (December 2007). "Natural genetic transformation: prevalence, mechanisms and function". Research in Microbiology (Amsterdam, the Netherlands: Elsevier for the Pasteur Institute) 158 (10): 767–778. doi:10.1016/j.resmic.2007.09.004. ISSN 0923-2508. PMID 17997281.
- ↑ 108.0 108.1 Bernstein, Bernstein & Michod 2012, pp. 1-50
- ↑ Ramesh, Marilee A.; Malik, Shehre-Banoo; Logsdon, John M., Jr. (January 26, 2005). "A Phylogenomic Inventory of Meiotic Genes: Evidence for Sex in Giardia and an Early Eukaryotic Origin of Meiosis" (PDF). Current Biology (Cambridge, MA: Cell Press) 15 (2): 185–191. doi:10.1016/j.cub.2005.01.003. ISSN 0960-9822. PMID 15668177. Retrieved 2008-12-22.
- ↑ 110.0 110.1 Otto, Sarah P.; Gerstein, Aleeza C. (August 2006). "Why have sex? The population genetics of sex and recombination". Biochemical Society Transactions (London: Portland Press on behalf of the Biochemical Society) 34 (Pt 4): 519–522. doi:10.1042/BST0340519. ISSN 0300-5127. PMID 16856849. Retrieved 2008-12-22.
- ↑ Hanley, Kathryn A.; Fisher, Robert N.; Case, Ted J. (June 1995). "Lower Mite Infestations in an Asexual Gecko Compared With Its Sexual Ancestors". Evolution (Hoboken, NJ: John Wiley & Sons on behalf of the Society for the Study of Evolution) 49 (3): 418–426. doi:10.2307/2410266. ISSN 0014-3820.
- ↑ Parker, Matthew A. (September 1994). "Pathogens and sex in plants". Evolutionary Ecology (Dordrecht, the Netherlands: Kluwer Academic Publishers) 8 (5): 560–584. doi:10.1007/bf01238258. ISSN 0269-7653.
- ↑ 113.0 113.1 Lin Dong; Shuhai Xiao; Bing Shen; Chuanming Zhou (January 2008). "Silicified Horodyskia and Palaeopascichnus from upper Ediacaran cherts in South China: tentative phylogenetic interpretation and implications for evolutionary stasis". Journal of the Geological Society (London: Geological Society of London) 165 (1): 367–378. doi:10.1144/0016-76492007-074. ISSN 0016-7649. Retrieved 2015-02-01.
- ↑ Birdsell & Wills 2003, pp. 27-137
- ↑ Bernstein, Hopf & Michod 1987, pp. 323–370
- ↑ Bell, Graham; Mooers, Arne O. (1997). "Size and complexity among multicellular organisms" (PDF). Biological Journal of the Linnean Society (Hoboken, NJ: John Wiley & Sons on behalf of the Linnean Society of London) 60 (3): 345–363. doi:10.1111/j.1095-8312.1997.tb01500.x. ISSN 0024-4074. Retrieved 2015-02-02.
- ↑ Kaiser, Dale (December 2001). "Building a multicellular organism". Annual Review of Genetics (Palo Alto, CA: Annual Reviews) 35: 103–123. doi:10.1146/annurev.genet.35.102401.090145. ISSN 0066-4197. PMID 11700279.
- ↑ 118.0 118.1 Nakagaki, Toshiyuki; Yamada, Hiroyasu; Tóth, Ágota (September 28, 2000). "Intelligence: Maze-solving by an amoeboid organism". Nature (London: Nature Publishing Group) 407 (6803): 470. doi:10.1038/35035159. ISSN 0028-0836. PMID 11028990. Retrieved 2008-09-03.
- ↑ Koschwanez, John H.; Foster, Kevin R.; Murray, Andrew W. (August 9, 2011). "Sucrose Utilization in Budding Yeast as a Model for the Origin of Undifferentiated Multicellularity". PLOS Biology (San Francisco, CA: Public Library of Science) 9 (8): e1001122. doi:10.1371/journal.pbio.1001122. ISSN 1545-7885. Retrieved 2015-02-02.
- ↑ 120.0 120.1 120.2 120.3 120.4 Butterfield, Nicholas J. (Summer 2000). "Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes". Paleobiology (Paleontological Society) 26 (3): 386–404. doi:10.1666/0094-8373(2000)026<0386:BPNGNS>2.0.CO;2. ISSN 0094-8373. Retrieved 2015-02-01.
- ↑ Dickey, Gwyneth (July 31, 2010). "Evidence for earlier multicellular life" (PNG). Science News (Washington, D.C.: Society for Science & the Public) 178 (3): 17. doi:10.1002/scin.5591780322. ISSN 0036-8423. Retrieved 2015-02-02.
- ↑ Gaidos, Eric J.; Dubuc, Timothy; Dunford, Mike et al. (December 2007). "The Precambrian emergence of animal life: a geobiological perspective". Geobiology (Hoboken, NJ: Wiley-Blackwell) 5 (4): 351–373. doi:10.1111/j.1472-4669.2007.00125.x. ISSN 1472-4677.
- ↑ Davidson, Michael W. (May 26, 2005). "Animal Cell Structure". Molecular Expressions. Tallahassee, FL: Florida State University. Retrieved 2008-09-03.
- ↑ Saupe, Stephen G. (January 3, 2004). "Concepts of Biology". Concepts of Biology (BIOL116) (Lecture). St. Joseph, MN: College of Saint Benedict and Saint John's University. Retrieved 2008-09-03.
- ↑ Hinde 2001, pp. 28–57
- ↑ Jun-Yuan Chen; Oliveri, Paola; Feng Gao et al. (August 1, 2002). "Precambrian Animal Life: Probable Developmental and Adult Cnidarian Forms from Southwest China" (PDF). Developmental Biology (Amsterdam, the Netherlands: Elsevier) 248 (1): 182–196. doi:10.1006/dbio.2002.0714. ISSN 0012-1606. PMID 12142030. Retrieved 2015-02-04.
- ↑ Grazhdankin, Dima (June 2004). "Patterns of distribution in the Ediacaran biotas: facies versus biogeography and evolution". Paleobiology (Paleontological Society) 30 (2): 203–221. doi:10.1666/0094-8373(2004)030<0203:PODITE>2.0.CO;2. ISSN 0094-8373.
- ↑ Seilacher, Adolf (August 1992). "Vendobionta and Psammocorallia: lost constructions of Precambrian evolution". Journal of the Geological Society (London: Geological Society of London) 149 (4): 607–613. doi:10.1144/gsjgs.149.4.0607. ISSN 0016-7649. Retrieved 2015-02-04.
- ↑ Martin, Mark W.; Grazhdankin, Dmitriy V.; Bowring, Samuel A. et al. (May 5, 2000). "Age of Neoproterozoic Bilaterian Body and Trace Fossils, White Sea, Russia: Implications for Metazoan Evolution". Science (Washington, D.C.: American Association for the Advancement of Science) 288 (5467): 841–845. Bibcode:2000Sci...288..841M. doi:10.1126/science.288.5467.841. ISSN 0036-8075. PMID 10797002. Retrieved 2015-02-05.
- ↑ Fedonkin, Mikhail A.; Waggoner, Benjamin M. (August 28, 1997). "The late Precambrian fossil Kimberella is a mollusc-like bilaterian organism". Nature (London: Nature Publishing Group) 388 (6645): 868–871. Bibcode:1997Natur.388..868F. doi:10.1038/42242. ISSN 0028-0836. Retrieved 2008-07-03.
- ↑ Mooi, Rich; David, Bruno (December 1998). "Evolution Within a Bizarre Phylum: Homologies of the First Echinoderms" (PDF). American Zoologist (Society for Integrative and Comparative Biology) 38 (6): 965–974. doi:10.1093/icb/38.6.965. ISSN 1540-7063. Retrieved 2015-02-05.
- ↑ McMenamin, Mark A. S. (September 2003). Spriggina is a trilobitoid ecdysozoan. Geoscience Horizons Seattle 2003. Abstracts with Programs 35 (6). Boulder, CO: Geological Society of America. p. 105. OCLC 249088612. Retrieved 2007-11-24. Paper No. 40-2 presented at the Geological Society of America's 2003 Seattle Annual Meeting (November 2–5, 2003) on November 2, 2003, at the Washington State Convention Center.
- ↑ Lin, J. P.; Gon, S. M.; Gehling, J. G.; Babcock, L. E.; Zhao, Y. L.; Zhang, X. L.; Hu, S. X.; Yuan, J. L.; Yu, M. Y.; Peng, J. (2006). "A Parvancorina-like arthropod from the Cambrian of South China". Historical Biology 18 (1): 33–45. doi:10.1080/08912960500508689.
- ↑ Butterfield, Nicholas J. (December 2006). "Hooking some stem-group 'worms': fossil lophotrochozoans in the Burgess Shale". BioEssays (Hoboken, NJ: John Wiley & Sons) 28 (12): 1161–1166. doi:10.1002/bies.20507. ISSN 0265-9247. PMID 17120226.
- ↑ 135.0 135.1 135.2 Bengtson 2004, pp. 67–78
- ↑ Gould 1989, pp. 124–136
- ↑ Gould 1989
- ↑ Budd, Graham E. (February 2003). "The Cambrian Fossil Record and the Origin of the Phyla" (PDF). Integrative and Comparative Biology (Oxford: Oxford University Press for the Society for Integrative and Comparative Biology) 43 (1): 157–165. doi:10.1093/icb/43.1.157. ISSN 1557-7023. PMID 21680420. Retrieved 2015-02-06.
- ↑ Budd, Graham E. (March 1996). "The morphology of Opabinia regalis and the reconstruction of the arthropod stem-group". Lethaia (Hoboken, NJ: Wiley-Blackwell) 29 (1): 1–14. doi:10.1111/j.1502-3931.1996.tb01831.x. ISSN 0024-1164.
- ↑ Marshall, Charles R. (May 2006). "Explaining the Cambrian 'Explosion' of Animals". Annual Review of Earth and Planetary Sciences (Palo Alto, CA: Annual Reviews) 34: 355–384. Bibcode:2006AREPS..34..355M. doi:10.1146/annurev.earth.33.031504.103001. ISSN 1545-4495. Retrieved 2015-02-06.
- ↑ Janvier, Philippe (2001). "Vertebrata (Vertebrates)". Encyclopedia of Life Sciences. Hoboken, NJ: John Wiley & Sons. doi:10.1038/npg.els.0001531. ISBN 0-470-01617-5. Retrieved 2015-01-21.
- ↑ Conway Morris, Simon (August 2, 2003). "Once we were worms". New Scientist (London: Reed Business Information) 179 (2406): 34. ISSN 0262-4079. Archived from the original on 2008-07-25. Retrieved 2008-09-05.
- ↑ Jun-Yuan Chen; Di-Ying Huang; Qing-Qing Peng et al. (July 8, 2003). "The first tunicate from the Early Cambrian of South China". Proc. Natl. Acad. Sci. U.S.A. (Washington, D.C.: National Academy of Sciences) 100 (14): 8314–8318. doi:10.1073/pnas.1431177100. ISSN 0036-8075. PMC 166226. PMID 12835415.
- ↑ D.-G. Shu; Conway Morris, Simon; J. Han et al. (January 30, 2003). "Head and backbone of the Early Cambrian vertebrate Haikouichthys". Nature (London: Nature Publishing Group) 421 (6922): 526–529. Bibcode:2003Natur.421..526S. doi:10.1038/nature01264. ISSN 0028-0836. PMID 12556891. Retrieved 2008-09-05.
- ↑ Sansom, Smith & Smith 2001, pp. 156–171
- ↑ 146.0 146.1 Cowen 2000, pp. 120–122
- ↑ 147.0 147.1 147.2 Selden 2001, "Terrestrialization of Animals," pp. 71–74
- ↑ Battistuzzi, F. U.; Feijao, A.; Hedges, S. B. (2004). "A genomic timescale of prokaryote evolution: insights into the origin of methanogenesis, phototrophy, and the colonization of land". BMC Evolutionary Biology 4: 44. doi:10.1186/1471-2148-4-44. PMC 533871. PMID 15535883.
- ↑ 149.0 149.1 149.2 149.3 149.4 149.5 149.6 Shear 2000, "The Early Development of Terrestrial Ecosystems," pp. 169–184
- ↑ Venturi, Sebastiano (September 2011). "Evolutionary Significance of Iodine". Current Chemical Biology (Sharjah, U.A.E.: Bentham Science Publishers) 5 (3): 155–162. doi:10.2174/187231311796765012. ISSN 1872-3136.
- ↑ Crockford, Susan J. (August 2009). "Evolutionary roots of iodine and thyroid hormones in cell-cell signaling". Integrative and Comparative Biology (Oxford: Oxford University Press for the Society for Integrative and Comparative Biology) 49 (2): 155–166. doi:10.1093/icb/icp053. ISSN 1557-7023. PMID 21669854.
- ↑ Venturi, Sebastiano; Donati, Francesco M.; Venturi, Alessandro; Venturi, Mattia (August 2000). "Environmental Iodine Deficiency: A Challenge to the Evolution of Terrestrial Life?". Thyroid (New Rochelle, NY: Mary Ann Liebert, Inc.) 10 (8): 727–729. doi:10.1089/10507250050137851. ISSN 1050-7256. PMID 11014322.
- ↑ Küpper, Frithjof C.; Carpenter, Lucy J.; McFiggans, Gordon B. et al. (May 13, 2008). "Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry". Proc. Natl. Acad. Sci. U.S.A. (Washington, D.C.: National Academy of Sciences) 105 (19): 6954–6958. Bibcode:2008PNAS..105.6954K. doi:10.1073/pnas.0709959105. ISSN 0036-8075. PMC 2383960. PMID 18458346.
- ↑ 154.0 154.1 Hawksworth, David L. (2002). "Lichens". Encyclopedia of Life Sciences. Hoboken, NJ: John Wiley & Sons. doi:10.1038/npg.els.0000368. ISBN 0-470-01617-5. Retrieved 2015-01-21.
- ↑ Retallack, Gregory J.; Feakes, Carolyn R. (January 2, 1987). "Trace Fossil Evidence for Late Ordovician Animals on Land". Science (Washington, D.C.: American Association for the Advancement of Science) 235 (4784): 61–63. Bibcode:1987Sci...235...61R. doi:10.1126/science.235.4784.61. ISSN 0036-8075. PMID 17769314.
- ↑ 156.0 156.1 Kenrick, Paul; Crane, Peter R. (September 4, 1997). "The origin and early evolution of plants on land" (PDF). Nature (London: Nature Publishing Group) 389 (6646): 33–39. Bibcode:1997Natur.389...33K. doi:10.1038/37918. ISSN 0028-0836. Retrieved 2015-02-10.
- ↑ Scheckler 2001, "Afforestation—the First Forests," pp. 67–70
- ↑ The phrase "Late Devonian wood crisis" is used at "Tetrapoda: Acanthostega". Palaeos. Retrieved 2015-02-10.
- ↑ Taylor, Thomas N.; Osborn, Jeffrey M. (February 1996). "The importance of fungi in shaping the paleoecosystem". Review of Palaeobotany and Palynology (Amsterdam, the Netherlands: Elsevier) 90 (3–4): 249–262. doi:10.1016/0034-6667(95)00086-0. ISSN 0034-6667. Retrieved 2015-02-10.
- ↑ Wilson, Heather M.; Anderson, Lyall I. (January 2004). "Morphology and taxonomy of Paleozoic millipedes (Diplopoda: Chilognatha: Archipolypoda) from Scotland". Journal of Paleontology (Paleontological Society) 78 (1): 169–184. doi:10.1666/0022-3360(2004)078<0169:MATOPM>2.0.CO;2. ISSN 0022-3360.
- ↑ Selden, Paul; Read, Helen J. (2008). "The Oldest Land Animals: Silurian Millipedes from Scotland" (PDF). Bulletin of the British Myriapod & Isopod Group (London: British Myriapod and Isopod Group) 23: 36–37. ISSN 1475-1739.
- ↑ Shear, William A.; Edgecombe, Gregory D. (March–May 2010). "The geological record and phylogeny of the Myriapoda". Arthropod Structure & Development (Amsterdam, the Netherlands: Elsevier) 39 (2–3): 174–190. doi:10.1016/j.asd.2009.11.002. ISSN 1467-8039. PMID 19944188.
- ↑ MacNaughton, Robert B.; Cole, Jennifer M.; Dalrymple, Robert W. et al. (May 2002). "First steps on land: Arthropod trackways in Cambrian-Ordovician eolian sandstone, southeastern Ontario, Canada". Geology (Boulder, CO: Geological Society of America) 30 (5): 391–394. Bibcode:2002Geo....30..391M. doi:10.1130/0091-7613(2002)030<0391:FSOLAT>2.0.CO;2. ISSN 0091-7613. Retrieved 2015-02-11.
- ↑ Vaccari, N. Emilio; Edgecombe, Gregory D.; Escudero, C. (July 29, 2004). "Cambrian origins and affinities of an enigmatic fossil group of arthropods". Nature (London: Nature Publishing Group) 430 (6999): 554–557. Bibcode:2004Natur.430..554V. doi:10.1038/nature02705. ISSN 0028-0836. PMID 15282604.
- ↑ Buatois, Luis A.; Mangano, M. Gabriela; Genise, Jorge F.; Taylor, Thomas N. (June 1998). "The Ichnologic Record of the Continental Invertebrate Invasion: Evolutionary Trends in Environmental Expansion, Ecospace Utilization, and Behavioral Complexity". PALAIOS (Tulsa, OK: SEPM Society for Sedimentary Geology) 13 (3): 217–240. doi:10.2307/3515447. ISSN 0883-1351. JSTOR 3515447. Retrieved 2015-02-11.
- ↑ Cowen 2000, p. 126
- ↑ Grimaldi & Engel 2005, pp. 155–160
- ↑ Grimaldi & Engel 2005, p. 12
- ↑ 169.0 169.1 169.2 Clack, Jennifer A. (December 2005). "Getting a Leg Up on Land". Scientific American (Stuttgart: Georg von Holtzbrinck Publishing Group) 293 (6): 100–107. doi:10.1038/scientificamerican1205-100. ISSN 0036-8733. PMID 16323697. Retrieved 2015-02-12.
- ↑ 170.0 170.1 Ahlberg, Per E.; Milner, Andrew R. (April 7, 1994). "The origin and early diversification of tetrapods". Nature (London: Nature Publishing Group) 368 (6471): 507–514. Bibcode:1994Natur.368..507A. doi:10.1038/368507a0. ISSN 0028-0836. Retrieved 2008-09-06.
- ↑ Gordon, Malcolm S.; Graham, Jeffrey B.; Wang, Tobias (September–October 2004). "Introduction to the Special Collection: Revisiting the Vertebrate Invasion of the Land". Physiological and Biochemical Zoology (Chicago, IL: University of Chicago Press on behalf of the Society for Integrative and Comparative Biology) 77 (5): 697–699. doi:10.1086/425182. ISSN 1522-2152.
- ↑ Daeschler, Edward B.; Shubin, Neil H.; Jenkins, Farish A., Jr. (April 6, 2006). "A Devonian tetrapod-like fish and the evolution of the tetrapod body plan" (PDF). Nature (London: Nature Publishing Group) 440 (7085): 757–763. Bibcode:2006Natur.440..757D. doi:10.1038/nature04639. ISSN 0028-0836. PMID 16598249. Retrieved 2015-02-12.
- ↑ deBraga, Michael; Rieppel, Olivier (July 1997). "Reptile phylogeny and the interrelationships of turtles". Zoological Journal of the Linnean Society (Hoboken, NJ: Wiley-Blackwell) 120 (3): 281–354. doi:10.1111/j.1096-3642.1997.tb01280.x. ISSN 1096-3642. Retrieved 2015-02-12.
- ↑ 174.0 174.1 Benton, Michael J.; Donoghue, Philip C. J. (January 2007). "Paleontological Evidence to Date the Tree of Life". Molecular Biology and Evolution (Oxford: Oxford University Press on behalf of the Society for Molecular Biology and Evolution) 24 (1): 26–53. doi:10.1093/molbev/msl150. ISSN 0737-4038. PMID 17047029. Retrieved 2015-02-12.
- ↑ 175.0 175.1 Benton, Michael J. (May 1990). "Phylogeny of the Major Tetrapod Groups: Morphological Data and Divergence Dates". Journal of Molecular Evolution (New York: Springer-Verlag) 30 (5): 409–424. doi:10.1007/BF02101113. ISSN 0022-2844. PMID 2111854. Retrieved 2015-02-12.
- ↑ Sidor, Christian A.; O'Keefe, F. Robin; Damiani, Ross et al. (April 14, 2005). "Permian tetrapods from the Sahara show climate-controlled endemism in Pangaea". Nature (London: Nature Publishing Group) 434 (7035): 886–889. Bibcode:2005Natur.434..886S. doi:10.1038/nature03393. ISSN 0028-0836. PMID 15829962. Retrieved 2008-09-08.
- ↑ Smith, Roger; Botha, Jennifer (September–October 2005). "The recovery of terrestrial vertebrate diversity in the South African Karoo Basin after the end-Permian extinction". Comptes Rendus Palevol (New York: Elsevier on behalf of the French Academy of Sciences) 4 (6–7): 623–636. doi:10.1016/j.crpv.2005.07.005. ISSN 1631-0683. Retrieved 2015-02-13.
- ↑ Benton 2005
- ↑ Sahney, Sarda; Benton, Michael J. (April 7, 2008). "Recovery from the most profound mass extinction of all time" (PDF). Proceedings of the Royal Society B (London: Royal Society) 275 (1636): 759–765. doi:10.1098/rspb.2007.1370. ISSN 0962-8452. PMC 2596898. PMID 18198148. Retrieved 2015-02-13.
- ↑ Gauthier et al. 1989, p. 345
- ↑ Benton, Michael J. (March 1983). "Dinosaur Success in the Triassic: A Noncompetitive Ecological Model" (PDF). The Quarterly Review of Biology (Stony Brook Foundation) 58 (1): 29–55. doi:10.1086/413056. ISSN 0033-5770. JSTOR 2828101. Retrieved 2008-09-08.
- ↑ 182.0 182.1 Padian 2004, pp. 210–231
- ↑ Lian-hai Hou; Zhonghe Zhou; Martin, Larry D.; Feduccia, Alan (October 19, 2002). "A beaked bird from the Jurassic of China". Nature (London: Nature Publishing Group) 377 (6550): 616–618. Bibcode:1995Natur.377..616H. doi:10.1038/377616a0. ISSN 0028-0836. Retrieved 2008-09-08.
- ↑ Clarke, Julia A.; Zhonghe Zhou; Fucheng Zhang (March 2006). "Insight into the evolution of avian flight from a new clade of Early Cretaceous ornithurines from China and the morphology of Yixianornis grabaui". Journal of Anatomy (Hoboken, NJ: John Wiley & Sons) 208 (3): 287–308. doi:10.1111/j.1469-7580.2006.00534.x. ISSN 1469-7580. PMC 2100246. PMID 16533313. Retrieved 2015-02-15.
- ↑ Ruben, John; Jones, Terry D. (August 2000). "Selective Factors Associated with the Origin of Fur and Feathers". American Zoologist (Society for Integrative and Comparative Biology) 40 (4): 585–596. doi:10.1093/icb/40.4.585. ISSN 1540-7063. Retrieved 2015-02-16.
- ↑ Zhe-Xi Luo; Crompton, Alfred W.; Ai-Lin Sun (May 25, 2001). "A New Mammaliaform from the Early Jurassic and Evolution of Mammalian Characteristics". Science (Washington, D.C.: American Association for the Advancement of Science) 292 (5521): 1535–1540. Bibcode:2001Sci...292.1535L. doi:10.1126/science.1058476. ISSN 0036-8075. PMID 11375489. Retrieved 2015-02-16.
- ↑ Cifelli, Richard L. (November 2001). "Early mammalian radiations". Journal of Paleontology (Paleontological Society) 75 (6): 1214–1226. doi:10.1666/0022-3360(2001)075<1214:EMR>2.0.CO;2. ISSN 0022-3360. Retrieved 2015-02-16.
- ↑ Flynn, John; Parrish, J. Michael; Rakotosamimanana, Berthe et al. (September 2, 1999). "A Middle Jurassic mammal from Madagascar". Nature (London: Nature Publishing Group) 401 (6748): 57–60. Bibcode:1999Natur.401...57F. doi:10.1038/43420. ISSN 0028-0836. Retrieved 2008-09-08.
- ↑ MacLeod, Norman; Rawson, Peter F.; Forey, Peter L. et al. (April 1997). "The Cretaceous–Tertiary biotic transition". Journal of the Geological Society (London: Geological Society of London) 154 (2): 265–292. doi:10.1144/gsjgs.154.2.0265. ISSN 0016-7649. Retrieved 2015-02-16.
- ↑ Alroy, John (March 1999). "The Fossil Record of North American Mammals: Evidence for a Paleocene Evolutionary Radiation". Systematic Biology (Oxford: Oxford University Press on behalf of the Society of Systematic Biologists) 48 (1): 107–118. doi:10.1080/106351599260472. ISSN 1063-5157. PMID 12078635.
- ↑ Archibald, J. David; Deutschman, Douglas H. (June 2001). "Quantitative Analysis of the Timing of the Origin and Diversification of Extant Placental Orders" (PDF). Journal of Mammalian Evolution (New York: Kluwer Academic Publishers/Plenum Publishers on behalf of the Society for Study of Mammalian Evolution) 8 (2): 107–124. doi:10.1023/A:1011317930838. ISSN 1064-7554. Retrieved 2015-02-16.
- ↑ Simmons, Nancy B.; Seymour, Kevin L.; Habersetzer, Jörg; Gunnell, Gregg F. (February 14, 2008). "Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation". Nature (London: Nature Publishing Group) 451 (7180): 818–821. Bibcode:2008Natur.451..818S. doi:10.1038/nature06549. ISSN 0028-0836. PMID 18270539.
- ↑ Thewissen, Madar & Hussain 1996
- ↑ 194.0 194.1 194.2 194.3 Crane, Friis and Pedersen 2000, "The Origin and Early Diversification of Angiosperms," pp. 233–250
- ↑ 195.0 195.1 195.2 195.3 Crepet, William L. (November 21, 2000). "Progress in understanding angiosperm history, success, and relationships: Darwin's abominably 'perplexing phenomenon'". Proc. Natl. Acad. Sci. U.S.A. (Washington, D.C.: National Academy of Sciences) 97 (24): 12939–12941. Bibcode:2000PNAS...9712939C. doi:10.1073/pnas.97.24.12939. ISSN 0036-8075. PMC 34068. PMID 11087846. Retrieved 2008-09-09.
- ↑ "evolution: plant timeline". Encyclopædia Britannica Online. Encyclopædia Britannica, Inc. 1996. OCLC 42796406. Retrieved 2015-02-16.
- ↑ Hughes, William O. H.; Oldroyd, Benjamin P.; Beekman, Madeleine; Ratnieks, Francis L. W. (May 30, 2008). "Ancestral Monogamy Shows Kin Selection Is Key to the Evolution of Eusociality". Science (Washington, D.C.: American Association for the Advancement of Science) 320 (5880): 1213–1216. Bibcode:2008Sci...320.1213H. doi:10.1126/science.1156108. ISSN 0036-8075. PMID 18511689. Retrieved 2015-02-17.
- ↑ Lovegrove, Barry G. (January 1991). "The evolution of eusociality in molerats (Bathyergidae): a question of risks, numbers, and costs". Behavioral Ecology and Sociobiology (Berlin: Springer-Verlag) 28 (1): 37–45. doi:10.1007/BF00172137. ISSN 0340-5443.
- ↑ Labandeira & Eble 1999
- ↑ Montgomery, Stephen (2009). "Darwin & the Descent of Man". Charles Darwin & Evolution. Cambridge: Christ's College. Retrieved 2015-02-17.
- ↑ Brunet, Michel; Guy, Franck; Pilbeam, David et al. (July 11, 2002). "A new hominid from the Upper Miocene of Chad, Central Africa". Nature (London: Nature Publishing Group) 418 (6894): 145–151. doi:10.1038/nature00879. ISSN 0028-0836. PMID 12110880. Retrieved 2008-09-09.
- ↑ de Heinzelin, Jean; Clark, J. Desmond; White, Tim et al. (April 23, 1999). "Environment and Behavior of 2.5-Million-Year-Old Bouri Hominids". Science (Washington, D.C.: American Association for the Advancement of Science) 284 (5414): 625–629. doi:10.1126/science.284.5414.625. ISSN 0036-8075. PMID 10213682. Retrieved 2015-02-19.
- ↑ De Miguel, Carmen; Henneberg, Maciej (2001). "Variation in hominid brain size: How much is due to method?". HOMO - Journal of Comparative Human Biology (Elsevier) 52 (1): 3–58. doi:10.1078/0018-442X-00019. ISSN 1618-1301.
- ↑ Leakey 1994, pp. 87–89
- ↑ Mellars, Paul (June 20, 2006). "Why did modern human populations disperse from Africa ca. 60,000 years ago? A new model". Proc. Natl. Acad. Sci. U.S.A. (Washington, D.C.: National Academy of Sciences) 103 (25): 9381–9386. Bibcode:2006PNAS..103.9381M. doi:10.1073/pnas.0510792103. ISSN 0027-8424. PMC 1480416. PMID 16772383. Retrieved 2015-02-20.
- ↑ Benton 2005a, Chapter 6: "Tetrapods of the Triassic"
- ↑ 208.0 208.1 MacLeod, Norman (January 6, 2001). "Extinction!". FirstScience.com. Retrieved 2015-02-20.
- ↑ Martin, Ronald E. (June 1995). "Cyclic and secular variation in microfossil biomineralization: clues to the biogeochemical evolution of Phanerozoic oceans". Global and Planetary Change (Amsterdam, the Netherlands: Elsevier) 11 (1–2): 1–23. Bibcode:1995GPC....11....1M. doi:10.1016/0921-8181(94)00011-2. ISSN 0921-8181.
- ↑ Martin, Ronald E. (June 1996). "Secular Increase in Nutrient Levels through the Phanerozoic: Implications for Productivity, Biomass, and Diversity of the Marine Biosphere". PALAIOS (Tulsa, OK: SEPM Society for Sedimentary Geology) 11 (3): 209–219. doi:10.2307/3515230. ISSN 0883-1351. JSTOR 3515230.
- ↑ 211.0 211.1 Rohde, Robert A.; Muller, Richard A. (March 10, 2005). "Cycles in fossil diversity" (PDF). Nature (London: Nature Publishing Group) 434 (7030): 208–210. Bibcode:2005Natur.434..208R. doi:10.1038/nature03339. ISSN 0028-0836. PMID 15758998. Retrieved 2008-09-22.
Bibliography
- Arrhenius, Svante (1980) [Arrhenius paper originally published 1903]. "The Propagation of Life in Space". In Goldsmith, Donald. The Quest for Extraterrestrial Life: A Book of Readings. Foreword by Sir Fred Hoyle. Mill Valley, CA: University Science Books. Bibcode:1980qel..book...32A. ISBN 0-935702-02-4. LCCN 79057423. OCLC 7121102.
- Bengtson, Stefan (2004). "Early Skeletal Fossils" (PDF). In Lipps, Jere H.; Waggoner, Benjamin M. Neoproterozoic-Cambrian Biological Revolutions: Presented as a Paleontological Society Short Course at the Annual Meeting of the Geological Society of America, Denver, Colorado, November 6, 2004. Paleontological Society Papers 10. New Haven, CT: Yale University Reprographics & Imaging Service; Paleontological Society. ISSN 1089-3326. OCLC 57481790. Retrieved 2015-02-06.
- Bennett, Jeffrey O. (2008). Beyond UFOs: The Search for Extraterrestrial Life and Its Astonishing Implications for Our Future. Princeton, NJ: Princeton University Press. ISBN 978-0-691-13549-6. LCCN 2007037872. OCLC 172521761.
- Benton, Michael J. (1997). Vertebrate Palaeontology (2nd ed.). London: Chapman & Hall. ISBN 0-412-73800-7. OCLC 37378512.
- Benton, Michael J. (2005) [Originally published 2003]. When Life Nearly Died: The Greatest Mass Extinction of All Time (1st paperback ed.). London: Thames & Hudson. ISBN 978-0-500-28573-2. LCCN 2002109744. OCLC 62145244.
- Benton, Michael J. (2005a). Vertebrate Palaeontology (3rd ed.). Malden, MA: Blackwell Science. ISBN 0-632-05637-1. LCCN 2003028152. OCLC 53970617.
- Bernstein, Harris; Bernstein, Carol; Michod, Richard E. (2012). "DNA Repair as the Primary Adaptive Function of Sex in Bacteria and Eukaryotes". In Kimura, Sakura; Shimizu, Sora. DNA Repair: New Research. Hauppauge, NY: Nova Science Publishers. ISBN 978-1-62100-808-8. LCCN 2011038504. OCLC 828424701.
- Bernstein, Harris; Hopf, Frederic A.; Michod, Richard E. (1987). "The Molecular Basis of the Evolution of Sex". In Scandalios, John G. Molecular Genetics of Development. Advances in Genetics. San Diego, CA: Academic Press. ISBN 0-12-017624-6. ISSN 0065-2660. OCLC 646754753. PMID 3324702.
- Birdsell, John A.; Wills, Christopher (2003). "The Evolutionary Origin and Maintenance of Sexual Recombination: A Review of Contemporary Models". In MacIntyre, Ross J.; Clegg, Michael T. Evolutionary Biology. Evolutionary Biology 33. New York: Springer Science+Business Media. ISBN 978-1-4419-3385-0. ISSN 0071-3260. OCLC 751583918.
- Briggs, Derek E. G.; Crowther, Peter R., eds. (2001). Palaeobiology II. Foreword by E. N. K. Clarkson. Malden, MA: Blackwell Science. ISBN 0-632-05149-3. LCCN 0632051477. OCLC 43945263.
- Cairns-Smith, A. G. (1968). "An Approach to a Blueprint for a Primitive Organism". In Waddington, C. H. Towards a Theoretical Biology 1. Edinburgh, Scotland: Edinburgh University Press. ISBN 0-85224-018-X. LCCN 71419832. OCLC 230043266.
- Clancy, Paul; Brack, André; Horneck, Gerda (2005). Looking for Life, Searching the Solar System. Cambridge; New York: Cambridge University Press. ISBN 0-521-82450-8. LCCN 2006271630. OCLC 57574490.
- Cowen, Richard (2000). History of Life (3rd ed.). Malden, MA: Blackwell Science. ISBN 0-632-04444-6. LCCN 99016542. OCLC 47011068.
- Dalrymple, G. Brent (1991). The Age of the Earth. Stanford, CA: Stanford University Press. ISBN 0-8047-1569-6. LCCN 90047051. OCLC 22347190.
- Futuyma, Douglas J. (2005). Evolution. Sunderland, MA: Sinauer Associates. ISBN 0-87893-187-2. LCCN 2004029808. OCLC 57311264.
- Gauthier, Jacques; Cannatella, David C.; de Queiroz, Kevin et al. (1989). "Tetrapod phylogeny". In Fernholm, Bo; Bremer, Kåre; Jörnvall, Hans. The Hierarchy of Life: Molecules and Morphology in Phylogenetic Analysis. International Congress Series 824. Amsterdam, the Netherlands; New York: Excerpta Medica/Elsevier Science Publishers B.V. (Biomedical Division). ISBN 0-444-81073-0. LCCN 89001132. OCLC 19129518. "Proceedings from Nobel Symposium 70 held at Alfred Nobel's Björkborn, Karlskoga, Sweden, August 29-September 2, 1988"
- Gee, Henry, ed. (2000). Shaking the Tree: Readings from Nature in the History of Life. Chicago, IL: University of Chicago Press. ISBN 0-226-28497-2. LCCN 99049796. OCLC 42476104.
- Gould, Stephen Jay (1989). Wonderful Life: The Burgess Shale and the Nature of History (1st ed.). New York: W. W. Norton & Company. ISBN 0-393-02705-8. LCCN 88037469. OCLC 18983518.
- Grimaldi, David; Engel, Michael S. (2005). Evolution of the Insects. Cambridge; New York: Cambridge University Press. ISBN 0-521-82149-5. LCCN 2004054605. OCLC 56057971.
- Hinde, Rosalind T. (2001). "The Cnidaria and Ctenophora". In Anderson, D. T. Invertebrate Zoology (2nd ed.). Melbourne; New York: Oxford University Press. ISBN 0-19-551368-1. LCCN 2002276846. OCLC 49663129.
- Holmes, Randall K.; Jobling, Michael G. (1996). "Genetics". In Baron, Samuel. Medical Microbiology (4th ed.). Galveston, TX: University of Texas Medical Branch. Exchange of Genetic Information. ISBN 0-9631172-1-1. LCCN 95050499. OCLC 33838234. PMID 21413277. Retrieved 2015-01-24.
- Krumbein, Wolfgang E.; Brehm, Ulrike; Gerdes, Gisela et al. (2003). "Biofilm, Biodictyon, Biomat Microbialites, Oolites, Stromatolites Geophysiology, Global Mechanism, Parahistology" (PDF). In Krumbein, Wolfgang E.; Paterson, David M.; Zavarzin, Georgii A. Fossil and Recent Biofilms: A Natural History of Life on Earth. Dordrecht, the Netherlands: Kluwer Academic Publishers. ISBN 1-4020-1597-6. LCCN 2003061870. OCLC 52901566. Archived from the original on 2007-01-06. Retrieved 2008-07-09.
- Labandeira, Conrad C.; Eble, Gunther J. (1999). "The Fossil Record of Insect Diversity and Disparity" (PDF). In Anderson, John M.; Thackeray, John Francis et al. Towards Gondwana Alive: Promoting biodiversity & stemming the Sixth Extinction. Pretoria: Gondwana Alive Society. ISBN 1-919795-43-X. LCCN 2001385090. OCLC 44822625. "Preview booklet for 'Gondwana alive : biodiversity and the evolving terrestrial biosphere', book planned for September 2000, and associated projects."
- Leakey, Richard (1994). The Origin of Humankind. Science Masters Series. New York: Basic Books. ISBN 0-465-03135-8. LCCN 94003617. OCLC 30739453.
- Margulis, Lynn (1981). Symbiosis in Cell Evolution: Life and its Environment on the Early Earth. San Francisco, CA: W. H. Freeman and Company. ISBN 0-7167-1256-3. LCCN 80026695. OCLC 6982472.
- Miller, G. Tyler; Spoolman, Scott E. (2012). Environmental Science (14th ed.). Belmont, CA: Brooks/Cole. ISBN 978-1-111-98893-7. LCCN 2011934330. OCLC 741539226.
- Newman, William L. (July 9, 2007). "Age of the Earth". Geologic Time. Reston, VA: Publications Services, USGS. OCLC 18792528. Retrieved 2008-08-29.
- O'Leary, Margaret R. (2008). Anaxagoras and the Origin of Panspermia Theory. Bloomington, IN: iUniverse. ISBN 0-595-49596-6. OCLC 757322661.
- Padian, Kevin (2004). "Basal Avialae". In Weishampel, David B.; Dodson, Peter; Osmólska, Halszka. The Dinosauria (2nd ed.). Berkeley: University of California Press. ISBN 0-520-24209-2. LCCN 2004049804. OCLC 55000644.
- Sansom, Ivan J.; Smith, Moya M.; Smith, M. Paul (2001). "The Ordovician radiation of vertebrates". In Ahlberg, Per Erik. Major Events in Early Vertebrate Evolution: Palaeontology, phylogeny, genetics and development. Systematics Association special volume series 61. London; New York: Taylor & Francis. ISBN 0-415-23370-4. LCCN 00062919. OCLC 51667292.
- Stearns, Beverly Peterson; Stearns, Stephen C. (1999). Watching, from the Edge of Extinction. New Haven, CT: Yale University Press. ISBN 0-300-07606-1. LCCN 98034087. OCLC 47011675.
- Thewissen, J. G. M.; Madar, S. I.; Hussain, S. T. (1996). Ambulocetus natans, an Eocene cetacean (Mammalia) from Pakistan. Courier Forschungsinstitut Senckenberg 191. Frankfurt: Senckenbergische Naturforschende Gesellschaft. ISBN 3-929907-32-1. ISSN 0341-4116. LCCN 97151576. OCLC 36463214.
Further reading
- Dawkins, Richard (1989). The Selfish Gene (New ed.). Oxford; New York: Oxford University Press. ISBN 0-19-286092-5. LCCN 89016077. OCLC 20012195.
- Dawkins, Richard (2004). The Ancestor's Tale: A Pilgrimage to the Dawn of Life. Boston: Houghton Mifflin Company. ISBN 0-618-00583-8. LCCN 2004059864. OCLC 56617123.
- Ruse, Michael; Travis, Joseph, eds. (2009). Evolution: The First Four Billion Years. Foreword by Edward O. Wilson. Cambridge, MA: Belknap Press of Harvard University Press. ISBN 978-0-674-03175-3. LCCN 2008030270. OCLC 225874308.
- Smith, John Maynard; Szathmáry, Eörs (1997) [Originally published 1995; Oxford: W. H. Freeman/Spektrum]. The Major Transitions in Evolution. Oxford; New York: Oxford University Press. ISBN 0-19-850294-X. LCCN 94026965. OCLC 715217397.
External links
General information
- "Evolution". The Virtual Fossil Museum. Retrieved 2015-02-22. General information on evolution compiled by Roger Perkins
- "Understanding Evolution: your one-stop resource for information on evolution". University of California, Berkeley. Retrieved 2015-02-22.
- "Evolution Resources". Washington, D.C.: National Academies. Retrieved 2015-02-23.
- "Tree of Life". Retrieved 2015-02-23. Tree of life diagram by Neal Olander
- "Evolution". New Scientist. Retrieved 2015-02-23.
- Brain, Marshall. How Evolution Works at HowStuffWorks
- "Modern Theories of Evolution: An Introduction to the Concepts and Theories That Led to Our Current Understanding of Evolution". Palomar College. Retrieved 2015-02-23. Tutorial created by Dennis O'Neil
History of evolutionary thought
- van Wyhe, John (ed.). "The Complete Work of Charles Darwin Online". Retrieved 2015-02-23.
- Price, R. G. "Understanding Evolution: History, Theory, Evidence, and Implications". rationalrevolution.net. Retrieved 2015-02-23.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||

