Erythrosine
 | |
| Names | |
|---|---|
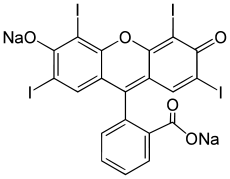
| IUPAC name
2-(6-Hydroxy-2,4,5,7-tetraiodo-3-oxo-xanthen-9-yl)benzoic acid | |
| Identifiers | |
| 16423-68-0 | |
| ChEMBL | ChEMBL1332616 |
| ChemSpider | 3144 |
| |
| Jmol-3D images | Image |
| PubChem | 3259 |
| |
| UNII | PN2ZH5LOQY |
| Properties | |
| C20H6I4Na2O5 | |
| Molar mass | 879.86 g/mol |
| Melting point | 142 to 144 °C (288 to 291 °F; 415 to 417 K)[1] |
| Hazards | |
| NFPA 704 | |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Erythrosine, also known as Red No. 3, is an organoiodine compound, specifically a derivative of fluorone. It is cherry-pink synthetic, primarily used for food coloring.[2] It is the disodium salt of 2,4,5,7-tetraiodofluorescein. Its maximum absorbance is at 530 nm[3] in an aqueous solution, and it is subject to photodegradation.
Uses
It is used as a:
- food coloring
- printing inks
- biological stain
- dental plaque disclosing agent
- radiopaque medium
- sensitizer for orthochromatic photographic films
Erythrosine is commonly used in sweets such as some candies and popsicles, and even more widely used in cake-decorating gels. It is also used to color pistachio shells.[4][5] As a food additive, it has the E number E127.
Health effects
As a result of efforts begun in the 1970s, in 1990 the U.S. FDA had instituted a partial ban on erythrosine, citing research that high doses have been found to cause cancer in rats.[6] In June 2008, the Center for Science in the Public Interest (CSPI) petitioned the FDA for a complete ban on erythrosine in the United States.[7]
A series of toxicology tests combined with a review of other reported studies concluded that erythrosine is non-genotoxic and the above-mentioned increase in tumors is caused by a non-genotoxic mechanism.[8]
A 1990 study concluded that "chronic erythrosine ingestion may promote thyroid tumor formation in rats via chronic stimulation of the thyroid by TSH." [9]
Regulation and prevalence
While commonly used in many countries of the world, erythrosine is less commonly used in the United States (the second least used after Fast Green FCF) because Allura Red AC (Red #40) is generally used instead. However, Allura Red AC is banned in many European countries because it is an azo dye.[10] Erythrosine can be used in colored food in USA without any restriction.[11]
Synonyms
Erythrosine B; Erythrosin B; Acid Red 51; C.I. 45430; FD & C Red No.3; E127; 2',4',5',7'-Tetraiodo-3',6'-dihydroxy-spiro[3H-isobenzofuran-1,9'-xanthen]-3-one disodium salt; Tetraiodofluorescein Sodium Salt; Calcoid Erythrosine N; 2,4,5,7-Tetraiodo-3,6-dihydroxyxanthene-9-spiro-1'-3H-isobenzofuran-3'-one disodium salt; 2',4',5',7'-Tetraiodofluorescein, disodium salt; C.I.Food Red 14; Aizen Erythrosine; Tetraiodifluorescein, disodium salt; Spiro[isobenzofuran- 1(3H),9'-[9H]xanthen]-3-one, 3',6'-dihydroxy-2',4',5',7'-tetraiodo-, disodium salt.[12][13]
Classification
It is listed under the following number systems:
- FD&C Red No. 3
- E number E127 (Food Red 14)
- Color Index no. 45430 (Acid Red 51)
- Indian Standards No. 1697
References
- ↑ http://www.chemicalbook.com/ChemicalProductProperty_US_CB0393696.aspx
- ↑ Phyllis A. Lyday "Iodine and Iodine Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim
- ↑ Food Color Additives
- ↑ Food Product Design Article
- ↑ Blue Diamond Ultra Premium Blend Mixed Nuts, distributed by Diamond Foods, Inc. Stockton, CA
- ↑ FDA: Red Dye's Reluctant Regulator; Partial Ban Points to Limitations of 30-Year-Old Delaney Clause, The Washington Post, February 7, 1990
- ↑ FDA Urged To Ban Some Food Dyes, CBS News, June 3, 2008
- ↑ Lin, George H. Y.; Brusick, David J. (1986). "Mutagenicity studies on FD&C Red No.3". Mutagenesis 1 (4): 253–259. doi:10.1093/mutage/1.4.253. PMID 2457780.
- ↑ Jennings, A. S.; Schwartz S. L.; Balter N. J.; Gardner D; Witorsch R. J. (May 1990). "Effects of oral erythrosine (2',4',5',7'-tetraiodofluorescein) on the pituitary-thyroid axis in rats.". Toxicology and Applied Pharmacology 103 (3): 549–56. PMID 2160137. Retrieved 13 May 2014.
- ↑ European Ban on Certain Azo Dyes
- ↑ CFR - Code of Federal Regulations Title 21, Sec. 74.303 FD+C Red No. 3.
- ↑ Erythrosin B, University of South Carolina
- ↑ Erythrosine, chemicalland21.com
