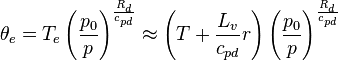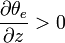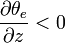Equivalent potential temperature
Equivalent potential temperature, commonly referred to as theta-e 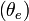 , is a quantity related to the stability of a column of air in the atmosphere.
, is a quantity related to the stability of a column of air in the atmosphere.
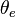 is the temperature a parcel of air would reach if all the water vapor in the parcel were to condense, releasing its latent heat, and the parcel was brought adiabatically to a standard reference pressure, usually 1000 hPa (1000 mbar) which is roughly equal to atmospheric pressure at sea level. In stable conditions,
is the temperature a parcel of air would reach if all the water vapor in the parcel were to condense, releasing its latent heat, and the parcel was brought adiabatically to a standard reference pressure, usually 1000 hPa (1000 mbar) which is roughly equal to atmospheric pressure at sea level. In stable conditions, 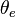 increases with altitude. If
increases with altitude. If 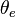 decreases with height, convection can occur. The comparison of the equivalent potential temperature of parcels of air at different pressures thus provides a measure of the instability of the column of air.[1]
decreases with height, convection can occur. The comparison of the equivalent potential temperature of parcels of air at different pressures thus provides a measure of the instability of the column of air.[1]
Explanation
Stability
Cool air is denser than warm air at the same pressure (see gas laws). Like a ball balanced on top of a hill, denser fluid lying above less dense fluid is dynamically unstable: if cool air is positioned above warm air, the former will sink and the latter will rise, the two volumes of air passing around and through each other, and perhaps mixing to some extent, until a stable condition (with denser fluid below and lighter fluid above) is achieved. The temperature near the ceiling of a room is consistently warmer than that near the floor.
If a hydrostatic fluid is compressible, the criterion for dynamic stability is not simply that denser fluid must lie below light fluid, but that small perturbations must tend to correct themselves. When lower fluid is raised up into upper fluid, (during which process the density of the lower fluid decreases due to the drop in pressure), stability requires that it remain denser than the upper fluid, so that gravity pulls it back toward its original position. The fluid is unstable if small perturbations tend to amplify themselves, i.e. if dense lower fluid, when displaced upward, expands enough to become lighter than the surrounding upper fluid, and therefore continues to move upward.
Potential temperature
In the atmosphere, where vertical variation in pressure is much larger than in a room, the situation is complicated by adiabatic temperature change: as a parcel of air moves upward, the ambient pressure drops, causing the parcel to expand. Some of the internal energy of the parcel is used up in doing the work required to expand against the atmospheric pressure, so the temperature of the parcel drops, even though it has not lost any heat. Conversely, a sinking parcel is compressed and becomes warmer even though no heat is added.
Air at the top of a mountain is usually colder than the air in the valley below, but the arrangement is not unstable: if a parcel of air from the valley were somehow lifted up to the top of the mountain, when it arrived it would be even colder than the air already there, due to adiabatic cooling; it would be heavier than the ambient air, and would sink back toward its original position. Similarly, if a parcel of cold mountain-top air were to make the trip down to the valley, it would arrive warmer and lighter than the valley air, and would float back up the mountain.
So cool air lying on top of warm air can be stable after all (as long as the temperature decrease with height is less than the adiabatic lapse rate); the dynamically important quantity is not the temperature, but the potential temperature—the temperature the air would have if it were brought adiabatically to a reference pressure. The air around the mountain is stable because the air at the top, due to its lower pressure, has a higher potential temperature than the warmer air below.
Water vapor
A parcel of air containing water vapor, if it rises far enough, cools to its dew point: it becomes saturated with water vapor. This occurs because the vapor pressure of water decreases at lower temperatures (see Clausius–Clapeyron relation). If the parcel of air continues to rise, water vapor begins to condense into liquid droplets. The condensing water releases its latent heat to the surrounding air, partially offsetting the adiabatic cooling. A saturated parcel of air therefore cools less than a dry one would as it rises (its temperature changes with height at the moist adiabatic lapse rate, which is smaller than the dry adiabatic lapse rate).
Saturated air can be unstable even though its potential temperature increases with height: if the warming due to condensation is enough that a parcel of saturated air which is displaced upward (and would otherwise cool to below the ambient temperature) ends up warmer (lighter) than the surrounding air, it will continue to rise. This is the reason for defining the equivalent potential temperature, in analogy with the potential temperature: potential temperature is a temperature adjusted for potential warming due to adiabatic compression; equivalent potential temperature factors in potential warming due to condensation as well. For saturated air, or for air which is likely to be lifted high enough to reach saturation, it is the equivalent potential temperature which must increase with height in order to ensure stability.
Formula
A number of approximate formulations are used for calculating equivalent potential temperature, since it is not easy to compute integrations along motion of the parcel. Bolton (1980) [2] gives review of such procedures with estimates of error. His best approximation formula is used when accuracy is needed:
Where:
-
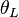 is (dry) potential temperature [K] at the lifted condensation level (LCL),
is (dry) potential temperature [K] at the lifted condensation level (LCL), -
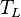 is (approximated) temperature [K] at LCL,
is (approximated) temperature [K] at LCL, -
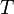 is temperature [K] of air at pressure
is temperature [K] of air at pressure 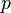 ,
, -
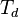 is dew point temperature at pressure
is dew point temperature at pressure 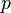 ,
, -
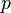 is pressure at the point [hPa or mbar],
is pressure at the point [hPa or mbar], -
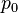 is standard reference pressure (1000 hPa),
is standard reference pressure (1000 hPa), -
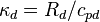 is the ratio of the specific gas constant to the specific heat of dry air at constant pressure (0.2854),
is the ratio of the specific gas constant to the specific heat of dry air at constant pressure (0.2854), -
 is mixing ratio of water vapor mass per mass [kg/kg] (sometimes value is given in [g/kg][3] and that should be divided by 1000).
is mixing ratio of water vapor mass per mass [kg/kg] (sometimes value is given in [g/kg][3] and that should be divided by 1000).
A little more theoretical formula is commonly used in literature like Holton (1972) [4] when theoretical explanation is important:
Where:
-
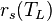 is saturated mixing ratio of water at temperature
is saturated mixing ratio of water at temperature 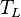 which is approximately considered same to specific humidity in low temperature,
which is approximately considered same to specific humidity in low temperature, -
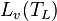 is latent heat of evaporation at temperature
is latent heat of evaporation at temperature 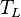 (2406 kJ/kg {at 40 °C} to 2501 kJ/kg {at 0 °C}), and
(2406 kJ/kg {at 40 °C} to 2501 kJ/kg {at 0 °C}), and -
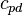 is specific heat of dry air at constant pressure (1005.7 J/(kg·K)).
is specific heat of dry air at constant pressure (1005.7 J/(kg·K)).
Further more simplified formula is used (in, for example, Stull 1988[5] §13.1 p. 546) for simplicity, if it is desirable to avoid computing 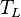 :
:
Where:
-
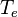 = equivalent temperature
= equivalent temperature -
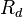 = specific gas constant for air (287.04 J/(kg·K))
= specific gas constant for air (287.04 J/(kg·K))
Usage

This applies on the synoptic scale for characterisation of air masses. For instance, in a study of the North American Ice Storm of 1998, professors Gyakum (McGill University, Montreal) and Roebber (Wisconsin University, Milwaukee) have demonstrated that the air masses involved originated from high Arctic at an altitude of 300 to 400 hPa the previous week, went down toward the surface as they moved to the Tropics, then moved back up along the Mississippi Valley toward the St. Lawrence Valley. The back trajectories were evaluated using the constant equivalent potential temperatures.[6]
In mesoscale, equivalent potential temperature is also a useful measure of the static stability of the unsaturated atmosphere. Under normal, stably stratified conditions, the potential temperature increases with height,
and vertical motions are suppressed. If the equivalent potential temperature decreases with height,
the atmosphere is unstable to vertical motions, and convection is likely. Situations in which the equivalent potential temperature decreases with height, indicating instability in saturated air, are quite common.
See also
Bibliography
- M K Yau and R.R. Rogers, Short Course in Cloud Physics, Third Edition, published by Butterworth-Heinemann, January 1, 1989, 304 pages. EAN 9780750632157 ISBN 0-7506-3215-1
References
- ↑ www.theweatherprediction.com
- ↑ D Bolton, 1980: The Computation of Equivalent Potential Temperature. Mon. Wea. Rev., Vol. 108, pp.1046-1053.
- ↑ Met Office. "Data processing procedure". E-AMDAR Evaluation. World Meteorological Organisation. Retrieved 2009-08-02.
- ↑ J R Holton, An Introduction to Dynamical Meteorology. Academic Press, 1972, 319 pages.
- ↑ R B Stull, An Introduction to Boundary Layer Meteorology, Kluwer, 1988, 666 pages, ISBN 9027727694.
- ↑ Gyakum, John R.; Roebber, Paul J. (December 2001). "The 1998 Ice Storm, Analysis of a Planetary-Scale Event" (pdf). Monthly Weather Review (American Meteorological Society) 129 (12): 2983–2997. Bibcode:2001MWRv..129.2983G. doi:10.1175/1520-0493(2001)129<2983:TISAOA>2.0.CO;2. Retrieved 19 June 2012..
![\theta_e = \theta_{L} \exp \left[ \left( \frac{3036}{T_L} - 1.78 \right) r \left(1 + 0.448 r\right)\right]](../I/m/73d34929e76756078a0f00fa2a17446b.png)
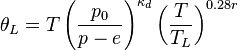
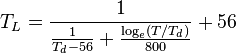
![\theta_e \approx \theta_L\exp\left[\frac{ r_s(T_L) L_v(T_L) }{ c_{pd} T_L }\right]](../I/m/5ed5295ab3988e9e3f87981d690c127a.png)