Entropic force

In physics, an entropic force acting in a system is a phenomenological force resulting from the entire system's statistical tendency to increase its entropy, rather than from a particular underlying microscopic force.[1]
Mathematical formulation
In the canonical ensemble, the entropic force 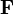 associated to a macrostate partition
associated to a macrostate partition 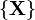 is given by:[2][3]
is given by:[2][3]
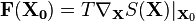
where 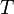 is the temperature,
is the temperature, 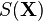 is the entropy associated to the macrostate
is the entropy associated to the macrostate 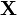 and
and 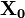 is the present macrostate.
is the present macrostate.
Examples
Brownian Motion
Entropic approach to Brownian movement is initially proposed by R. M. Neumann,[2][4] Neumann derived the entropic force for a particle undergoing three-dimensional Brownian motion using Boltzmann equation, denoting this force as a diffusional driving force or radial force. In the paper, three example systems are shown to exhibit such a force electrostatic system of molten salt, surface tension and rubber elasticity.
Polymers
A standard example of an entropic force is the elasticity of a freely-jointed polymer molecule described by a Gaussian distribution.[4] If the molecule is pulled into an extended configuration, the system has an increased amount of predictability. But randomly coiled configurations are overwhelmingly more probable; i.e., they have greater entropy. This results in the chain eventually returning (through diffusion) to such a configuration. To the macroscopic observer, the precise origin of the microscopic forces that drive the motion is irrelevant. The observer simply sees the polymer contract into a state of higher entropy, as if driven by an elastic force.
Hydrophobic force

Another example of an entropic force is the hydrophobic force. At room temperature, it partly originates from the loss of entropy by the 3D network of water molecules when they interact with molecules of dissolved substance. Each water molecule is capable of
- donating two hydrogen bonds through the two protons
- accepting two more hydrogen bonds through the two sp3-hybridized lone pairs
Therefore, water molecules can form an extended three-dimensional network. Introduction of a non-hydrogen-bonding surface disrupts this network. The water molecules rearrange themselves around the surface, so as to minimize the number of disrupted hydrogen bonds. This is in contrast to hydrogen fluoride (which can accept 3 but donate only 1) or ammonia (which can donate 3 but accept only 1), which mainly form linear chains.
If the introduced surface had an ionic or polar nature, there would be water molecules standing upright on 1 (along the axis of an orbital for ionic bond) or 2 (along a resultant polarity axis) of the four sp3 orbitals.[5] These orientations allow easy movement, i.e. degrees of freedom, and thus lowers entropy minimally. But a non-hydrogen-bonding surface with a moderate curvature forces the water molecule to sit tight on the surface, spreading 3 hydrogen bonds tangential to the surface, which then become locked in a clathrate-like basket shape. Water molecules involved in this clathrate-like basket around the non-hydrogen-bonding surface are constrained in their orientation. Thus, any event that would minimize such a surface is entropically favored. For example, when two such hydrophobic particles come very close, the clathrate-like baskets surrounding them merge. This releases some of the water molecules into the bulk of the water, leading to an increase in entropy. This is the basis of the so-called "attraction" between hydrophobic objects in water.
Directional Entropic Force
Entropic forces also occur in the physics of gases and solutions, where they generate the pressure of an ideal gas (the energy of which depends only on its temperature, not its volume), the osmotic pressure of a dilute solution, and in colloidal suspensions, where they are responsible for the crystallization of hard spheres.
In nano and colloidal science, Entropic Forces usually come from the effect of depletion, where small particles induce crystallization of bigger ones.
Even in the absence of depletion, however, scientist Sharon Glotzer and collaborators recently conjectured that Directional Entropic Forces could be responsible for the alignment of facets observed prior to the assembly and/or crystallization of systems of polyhedral nano and colloidal particles.[6] This was later proven to be correct[7][8] and allowed for the development of a roadmap for the assembly of polyhedral particles into atomic isostructures.[9]
Speculative examples
In recent years (especially since 2009) some forces that are generally regarded as conventional forces have been argued to be actually entropic in nature. These theories remain speculative and are the subject of ongoing work.
Gravity
It is generally believed that gravity is a microscopic force (or arguably a pseudo-force in general relativity). However, in 2009, Erik Verlinde[10] argued that gravity can be explained as an entropic force.[11]
For example, when someone throws a ball in the air, it follows a parabolic trajectory (in the absence of wind resistance). Conventionally, it is said that the ball follows a deterministic path dictated by Newton's law of gravity or general relativity. However, in the entropic theory, it is argued that the ball can follow any trajectory and picks a trajectory "at random." A calculation demonstrates that, in the collection of possible trajectories, the overwhelming majority are almost exactly the same as the parabolic trajectory; therefore, the ball is observed to follow a parabola.
Other forces
Other fundamental forces have been argued recently to be entropic in origin, including Coulomb's law,[12][13][14] the electroweak and strong forces,[15] and dark matter and dark energy.[16]
Links to Occam's Razor
A formal simultaneous connection between the mathematical structure of the discovered laws of nature, intelligence and the entropy-like measures of complexity was previously noted in 2000 by Andrei Soklakov[17] in the context of Occam's razor principle.
See also
- Colloids
- Nanomechanics
- Data clustering
- Entropic elasticity of an ideal chain
References
- ↑ A history of thermodynamics: the doctrine of energy and entropy by Ingo Müller, p115
- ↑ 2.0 2.1 Neumann RM (1980). "Entropic approach to Brownian movement". American Journal of Physics 48 (5): 354. doi:10.1119/1.12095.
- ↑ On the origin of gravity and the laws of Newton, Erik Verlinde
- ↑ 4.0 4.1 Neumann RM (1977). "The entropy of a single Gaussian macromolecule in a noninteracting solvent". The Journal of Chemical Physics 66 (2): 870. doi:10.1063/1.433923.
- ↑ Encyclopedia of Life Science Article on Hydrophobic Effect; See Figure 4: http://xibalba.lcg.unam.mx/~rgalindo/bioquimica/BQPosgrado2011/I%20FQ%20repaso/HydrophobicEffect.pdf
- ↑ "Crystalline Assemblies and Densest Packings of a Family of Truncated Tetrahedra and the Role of Directional Entropic Forces" (PDF). ACS. Archived from the original on 2011-12-01. Retrieved 23 June 2012.
- ↑ "Unified Theoretical Framework for Shape Entropy in Colloids" (PDF). Archived from the original (PDF) on 2013-09-03. Retrieved 20 October 2013.
- ↑ "A Directional Entropic Force Approach to Assemble Anisotropic Nanoparticles into Superlattices" (PDF). Archived from the original (PDF) on 2013-09-03. Retrieved 13 Jan 2014.
- ↑ "Structural Diversity and the Role of Particle Shape and Dense Fluid Behavior in Assemblies of Hard Polyhedra" (PDF). Archived from the original (PDF) on 2012-02-10. Retrieved 23 June 2012.
- ↑ Verlinde, Eric (6 January 2010). "Title: On the Origin of Gravity and the Laws of Newton". arXiv:1001.0785 [hep-th].
- ↑ E.P. Verlinde. "On the Origin of Gravity and the Laws of Newton". JHEP 04, 29 (2011). arXiv:1001.0785. Bibcode:2011JHEP...04..029V. doi:10.1007/JHEP04(2011)029.
- ↑ http://arxiv.org//abs/1001.4965, Coulomb Force as an Entropic Force, T. Wang
- ↑ http://arxiv.org//abs/0809.4631, Simple field theoretical approach of Coulomb systems. Entropic effects, D. di Caprio, J.P. Badiali, M. Holovko
- ↑ http://arxiv.org//abs/1009.5561, Entropic Corrections to Coulomb's Law, A. Sheykhi, S. H. Hendi
- ↑ http://arxiv.org//abs/1008.4147,'' Emergent Gauge Fields, Peter G.O. Freund
- ↑ http://arxiv.org//abs/1009.1506 Unification of Dark Matter and Dark Energy in a Modified Entropic Force Model, Zhe Chang, Ming-Hua Li, Xin Li
- ↑ Andrei N. Soklakov, "Occam's razor as a formal basis for a physical theory" (arXiv:math-ph/0009007, September 2000; Foundations of Physics Letters, 2002), "Complexity analysis for algorithmically simple strings" (arXiv:cs/0009001, September 2000).
| ||||||||||||||||||||||||||||||||||||||||||||