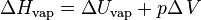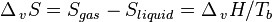Enthalpy of vaporization
.png)
The enthalpy of vaporization, (symbol ∆Hvap) also known as the (latent) heat of vaporization or heat of evaporation, is the enthalpy change required to transform a given quantity of a substance from a liquid into a gas at a given pressure (often atmospheric pressure, as in STP).
It is often measured at the normal boiling point of a substance; although tabulated values are usually corrected to 298 K, the correction is often smaller than the uncertainty in the measured value.
The heat of vaporization is temperature-dependent, though a constant heat of vaporization can be assumed for small temperature ranges and for reduced temperature Tr<<1.0. The heat of vaporization diminishes with increasing temperature and it vanishes completely at the critical temperature (Tr=1) because above the critical temperature the liquid and vapor phases no longer exist, since the substance is a supercritical fluid.
Units
Values are usually quoted in J/mol or kJ/mol (molar enthalpy of vaporization), although kJ/kg or J/g (specific heat of vaporization), and older units like kcal/mol, cal/g and Btu/lb are sometimes still used, among others.
Physical model for vaporization

A simple physical model for the liquid–gas phase transformation was proposed in 2009 by Jozsef Garai.[1] It is suggested that the energy required to free an atom from the liquid is equivalent to the energy needed to overcome the surface resistance of the liquid. The model allows calculating the latent heat by multiplying the maximum surface area covering an atom (Fig. 1) with the surface tension and the number of atoms in the liquid. The calculated latent heat of vaporization values for the investigated 45 elements agrees well with experiments. Another model which utilizes the data set from Jozsef Garai's model shows that the liquid–gas phase change can be explained in terms of kinetic theory by considering that the energy required for vaporization is extracted from all six of the vaporizing molecule's neighbours. This includes a required rethink of the probability of vaporization, and has consequences to the Clausius-Clapeyron equation. Moreover, it does resolve the issue of the latent heat of vaporization being significantly greater than the thermal energy exchanged between molecules, i.e. at boiling point the latent heat for water is approximately 13.2 times kT (Boltzmann's factor multiplied by boiling temperature.)[2]
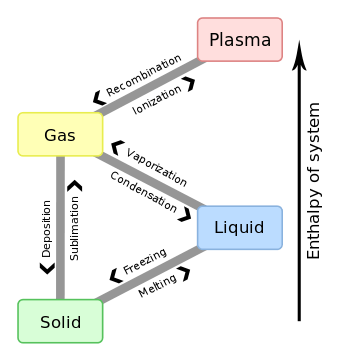
Enthalpy of condensation
The enthalpy of condensation (or heat of condensation) is by definition equal to the enthalpy of vaporization with the opposite sign: enthalpy changes of vaporization are always positive (heat is absorbed by the substance), whereas enthalpy changes of condensation are always negative (heat is released by the substance).
Thermodynamic background
The enthalpy of vaporization can be written as
It is equal to the increased internal energy of the vapor phase compared with the liquid phase, plus the work done against ambient pressure. The increase in the internal energy can be viewed as the energy required to overcome the intermolecular interactions in the liquid (or solid, in the case of sublimation). Hence helium has a particularly low enthalpy of vaporization, 0.0845 kJ/mol, as the van der Waals forces between helium atoms are particularly weak. On the other hand, the molecules in liquid water are held together by relatively strong hydrogen bonds, and its enthalpy of vaporization, 40.65 kJ/mol, is more than five times the energy required to heat the same quantity of water from 0 °C to 100 °C (cp = 75.3 J K−1 mol−1). Care must be taken, however, when using enthalpies of vaporization to measure the strength of intermolecular forces, as these forces may persist to an extent in the gas phase (as is the case with hydrogen fluoride), and so the calculated value of the bond strength will be too low. This is particularly true of metals, which often form covalently bonded molecules in the gas phase: in these cases, the enthalpy of atomization must be used to obtain a true value of the bond energy.
An alternative description is to view the enthalpy of condensation as the heat which must be released to the surroundings to compensate for the drop in entropy when a gas condenses to a liquid. As the liquid and gas are in equilibrium at the boiling point (Tb), ΔvG = 0, which leads to:
As neither entropy nor enthalpy vary greatly with temperature, it is normal to use the tabulated standard values without any correction for the difference in temperature from 298 K. A correction must be made if the pressure is different from 100 kPa, as the entropy of a gas is proportional to its pressure (or, more precisely, to its fugacity): the entropies of liquids vary little with pressure, as the compressibility of a liquid is small.
These two definitions are equivalent: the boiling point is the temperature at which the increased entropy of the gas phase overcomes the intermolecular forces. As a given quantity of matter always has a higher entropy in the gas phase than in a condensed phase (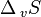 is always positive), and from
is always positive), and from
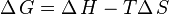 ,
,
the Gibbs free energy change falls with increasing temperature: gases are favored at higher temperatures, as is observed in practice.
Vaporization enthalpy of electrolyte solutions
Estimation of the enthalpy of vaporization of electrolyte solutions can be simply carried out using equations based on the chemical thermodynamic models, such as Pitzer model[3] or TCPC model.[4]
Selected values
Elements
| Enthalpies of vaporization of the elements | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Group → | ||||||||||||||||||
| ↓ Period | ||||||||||||||||||
| 1 | H 0.44936 |
He 0.0845 | ||||||||||||||||
| 2 | Li 145.92 |
Be 292.40 |
B 489.7 |
C 355.8 |
N 2.7928 |
O 3.4099 |
F 3.2698 |
Ne 1.7326 | ||||||||||
| 3 | Na 96.96 |
Mg 127.4 |
Al 293.4 |
Si 300 |
P 12.129 |
S 1.7175 |
Cl 10.2 |
Ar 6.447 | ||||||||||
| 4 | K 79.87 |
Ca 153.6 |
Sc 314.2 |
Ti 421 |
V 452 |
Cr 344.3 |
Mn 226 |
Fe 349.6 |
Co 376.5 |
Ni 370.4 |
Cu 300.3 |
Zn 115.3 |
Ga 258.7 |
Ge 330.9 |
As 34.76 |
Se 26.3 |
Br 15.438 |
Kr 9.029 |
| 5 | Rb 72.216 |
Sr 144 |
Y 363 |
Zr 581.6 |
Nb 696.6 |
Mo 598 |
Tc 660 |
Ru 595 |
Rh 493 |
Pd 357 |
Ag 250.58 |
Cd 100 |
In 231.5 |
Sn 295.8 |
Sb 77.14 |
Te 52.55 |
I 20.752 |
Xe 12.636 |
| 6 | Cs 67.74 |
Ba 142 |
|
Hf 575 |
Ta 743 |
W 824 |
Re 715 |
Os 627.6 |
Ir 604 |
Pt 510 |
Au 334.4 |
Hg 59.229 |
Tl 164.1 |
Pb 177.7 |
Bi 104.8 |
Po 60.1 |
At 27.2 |
Rn 16.4 |
| 7 | Fr n/a |
Ra 37 |
|
Rf n/a |
Db n/a |
Sg n/a |
Bh n/a |
Hs n/a |
Mt n/a |
Ds n/a |
Rg n/a |
Cn n/a |
Uut n/a |
Fl n/a |
Uup n/a |
Lv n/a |
Uus n/a |
Uuo n/a |
| |
La 414 |
Ce 414 |
Pr n/a |
Nd n/a |
Pm n/a |
Sm n/a |
Eu n/a |
Gd n/a |
Tb n/a |
Dy n/a |
Ho n/a |
Er n/a |
Tm n/a |
Yb n/a |
Lu n/a | |||
| |
Ac n/a |
Th 514.4 |
Pa n/a |
U n/a |
Np n/a |
Pu n/a |
Am n/a |
Cm n/a |
Bk n/a |
Cf n/a |
Es n/a |
Fm n/a |
Md n/a |
No n/a |
Lr n/a | |||
| Enthalpy in in kJ/mol, measured at their respective normal boiling points | ||||||||||||||||||
| 0–10 kJ/mol | 10–100 kJ/mol | 100–300 kJ/mol | >300 kJ/mol | |||||||||||||||
Other common substances
Enthalpies of vaporization of common substances, measured at their respective standard boiling points:
| Compound | Boiling Point at normal pressure | Heat of vaporization (kJ mol−1) |
Heat of vaporization (kJ kg−1) |
|---|---|---|---|
| Acetone | 329–330 K, 56–57 °C, 133–134 °F | 31.3 | 538.9 |
| Aluminium | 2792 K, 2519 °C, 4566 °F | 294.0 | 10500 |
| Ammonia | 240 K, −33.34 °C, −28 °F | 23.35 | 1371 |
| Butane | 272–274 K, −1 °C, 30–34 °F | 21.0 | 320 |
| Diethyl ether | 307.8 K, 34.6 °C, 94.3 °F | 26.17 | 353.1 |
| Ethanol | 352 K, 78.37 °C, 173 °F | 38.6 | 841 |
| Hydrogen | 20.271 K, -252.879 °C, -423.182 °F | 0.46 | 451.9 |
| Iron | 3134 K, 2862 °C, 5182 °F | 340 | 6090 |
| Isopropyl alcohol | 356 K, 82.6 °C, 181 °F | 44.0 | 732.2 |
| Methane | 109–113 K, −164—160 °C, −263—256 °F | 8.17 | 480.6 |
| Methanol | 338 K, 64.7 °C, 148 °F | 35.3 | 1104 |
| Propane | 230.9–231.11 K,-42—42 °C, −44—44 °F | 15.7 | 356 |
| Phosphine | 185 K, −87.7 °C, −126 °F | 14.6 | 429.4 |
| Water | 373.15 K, 100 °C, 212 °F | 40.65 | 2260 @ 98.64 °C [5] |
See also
- Enthalpy of fusion
- Enthalpy of sublimation
- Joback method (Estimation of the heat of vaporization at the normal boiling point from molecular structures)
References
- ↑ Garai, J. (2009). "Physical model for vaporization". Fluid Phase Equilibria 283: 89–77. doi:10.1016/j.fluid.2009.06.005.
- ↑ K. Mayhew, Phys. Essays 26, vol 4, 604 (2013)
- ↑ X. Ge, X. Wang. Estimation of Freezing Point Depression, Boiling Point Elevation and Vaporization enthalpies of electrolyte solutions. Ind. Eng. Chem. Res. 48(2009)2229–2235. http://pubs.acs.org/doi/abs/10.1021/ie801348c (Correction: 2009, 48, 5123)http://pubs.acs.org/doi/abs/10.1021/ie900434h
- ↑ X. Ge, X. Wang. Calculations of Freezing Point Depression, Boiling Point Elevation, Vapor Pressure and Enthalpies of Vaporization of Electrolyte Solutions by a Modified Three-Characteristic Parameter Correlation Model. J. Sol. Chem. 38(2009)1097–1117.http://www.springerlink.com/content/21670685448p5145/
- ↑ Çengel, Yunus A., and Michael A. Boles. "Appendix 1." Thermodynamics: An Engineering Approach. 7th ed. Boston: McGraw-Hill, 2011. 914. Print.
- CODATA Key Values for Thermodynamics
- Kugler HK & Keller C (eds) 1985, Gmelin handbook of inorganic and organometallic chemistry, 8th ed., 'At, Astatine', system no. 8a, Springer-Verlag, Berlin, ISBN 3-540-93516-9, pp. 116–117
- NIST Chemistry WebBook
- Sears, Zemansky et al., University Physics, Addison-Wesley Publishing Company, Sixth ed., 1982, ISBN 0-201-07199-1
| ||||||||||||||||||||||||||||||||||||