Endergonic
Endergonic (from the prefix endo-, derived from the Greek word ἔνδον endon, "within", and the Greek word ἔργον ergon, "work") means "absorbing energy in the form of work." Endergonic reactions are not spontaneous. By thermodynamic standards, positive work, a form of energy, is defined as moving from the surroundings (the external region) to the system (the internal region). Thus, an endergonic process, as contrasted with an exergonic process, is one wherein the system absorbs energy from the surroundings. As a result, during an endergonic process, energy is put into the system, if the transformation occurs at constant pressure and temperature, ∆G > 0. An endergonic reaction is a chemical reaction that absorbs energy in the form of work. A good example of a net endergonic process is photosynthesis. Also, in metabolism, an endergonic process is anabolic, meaning, that energy is stored. In metabolism, catabolic and anabolic processes are coupled by adenosine triphosphate (ATP).
All physical and chemical systems in the universe follow the second law of thermodynamics and proceed in a downhill, i.e., exergonic, direction. Thus, left to itself, any physical or chemical system will proceed, according to the second law of thermodynamics, in a direction that tends to lower the free energy of the system, and thus to expend energy in the form of work. These reactions occur spontaneously.
A chemical reaction is endergonic when non spontaneous. Thus in this type of reactions the Gibbs free energy increases. The entropy is included in any change of the Gibbs free energy. This differs from an endothermic reaction where the entropy is not included. The Gibbs free energy is calculated with the Gibbs-Helmholtz equation:
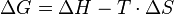
where:
T = Temperature in Kelvins (K)
ΔG = Change in the Gibbs free energy
ΔS = Change in entropy (at 298 K) as ΔS = Σ{S(Product)} - Σ{S(Reagent)}
ΔH = Change in enthalpy (at 298 K) as ΔH = Σ{H(Product)} - Σ{H(Reagent)}
A chemical reaction progresses non spontaneously when the Gibbs free energy increases, in that case the ΔG is positive. In exergonic reactions the ΔG is negative and in endergonic reactions the ΔG is positive:
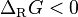 exergon
exergon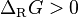 endergon
endergon
where:
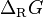 equals the change in the Gibbs free energy after completion of a chemical reaction.
equals the change in the Gibbs free energy after completion of a chemical reaction.
See also
|
|