Enadoline
 | |
| Systematic (IUPAC) name | |
|---|---|
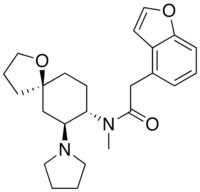
| 2-(1-Benzofuran-4-yl)-N-methyl-N-[(5R,7S,8S)-7-pyrrolidin-1-yl-1-oxaspiro[4.5]decan-8-yl]acetamide | |
| Clinical data | |
| Identifiers | |
|
124378-77-4 | |
| None | |
| PubChem | CID 60768 |
| IUPHAR ligand | 1646 |
| ChemSpider |
54765 |
| UNII |
KJL283326C |
| ChEMBL |
CHEMBL318859 |
| Chemical data | |
| Formula | C24H32N2O3 |
| 396.52 g/mol | |
|
SMILES
| |
| |
| | |
Enadoline is a drug which acts as a highly selective κ-opioid agonist.
In human studies, it produced visual distortions and feelings of dissociation, reminiscent of the effects of salvinorin A.[1]
It was studied as a potential analgesic, but abandoned because of the dose-limiting effects of dysphoria, which could be expected from a κ-opioid agonist. There was mention of its potential in treating comatose head injury or stroke victims, where that type of side effect would be immaterial.[2]
Potency
When enadoline was first reported in 1990, it was "the most potent κ-selective analgesic ever reported ... 25 times more potent than morphine and 17 times more potent than U-62066".[3]
See also
References
- ↑ Walsh SL, Strain EC, Abreu ME, Bigelow GE (2001). "Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans". Psychopharmacology (Berl.) 157 (2): 151–62. doi:10.1007/s002130100788. PMID 11594439.
- ↑ Barber A, Gottschlich R (1997). "Novel developments with selective, non-peptidic kappa-opioid receptor agonists". Expert Opin Investig Drugs 6 (10): 1351–68. doi:10.1517/13543784.6.10.1351. PMID 15989506.
- ↑ Halfpenny, Paul R. (1990). "Highly selective .kappa.-opioid analgesics. 3. Synthesis and structure-activity relationships of novel N-[2-(1-pyrrolidinyl)-4- or -5-substituted cyclohexyl]arylacetamide derivatives". Journal of Medicinal Chemistry 33 (1): 286–291. doi:10.1021/jm00163a047.
| ||||||||||||||||||||||||||||||||||||||