Emamectin
 | |
| Names | |
|---|---|
| Other names
4′′-Deoxy-4′′-epi-methylaminoavermectin B1; Epimethylamino-4′′-deoxyavermectin; MK 243; EMA; GWN 1972 | |
| Identifiers | |
| ATCvet code | QP54 |
| 119791-41-2 155569-91-8 (Benzoate) | |
| Jmol-3D images | Image |
| RTECS number | CL1203005 |
| |
| UNII | 8C43B81H4W |
| Properties | |
| Molecular formula |
C49H75NO13 |
| Molar mass | 886.12 g·mol−1 |
| Appearance | White or faintly yellow powder |
| Melting point | 141 °C (286 °F; 414 K) |
| 30-50 ppm (pH 7) | |
| Hazards | |
| R-phrases | R25 R36 R50 R57 R58 |
| S-phrases | S26 S36 S45 S60 S61 |
| NFPA 704 | |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
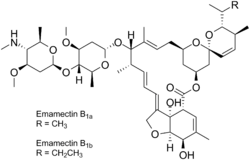
Emamectin is the 4”-deoxy-4”-methylamino derivative of abamectin, a 16-membered macrycyclic lactone produced by the fermentation of the soil actinomycete Streptomyces avermitilis.[1][2] It is generally prepared at the salt with benzoic acid, emamectin benzoate, which is a white or faintly yellow powder.[3] Emamectin is widely used in the US and Canada as an insecticide because of its chloride channel activation properties.[4]
History
Emamectin, produced by the bacterium Streptomyces avermitilis, belongs to the avermectin family of compounds all of which exhibit toxicity for nematodes, arthropods, and several other pests. The benzoate salt of emamectin in particular has found widespread use as an insecticide and is approved by the EPA for use in prevention of emerald ash borer in ash trees.[5] Emamectin is derived from avermectin B1, also known as abamectin, a mixture of the natural avermectin B1a and B1b. Emamectin has also shown promising applications in the eradication of fish lice and in fish farming.[6]
Preparation
Emamectin is derived from abamectin by replacement of an epi-amino-methyl (NHCH3) group by a hydroxyl (-OH) group at the 4”-position. Emamectin, like abamectin, is a mixture of two homologue compounds termed B1a and B1b which differ on the C-25 side-chain by one methylene (CH2) group. B1a contains a sec-butyl group while B1b has an isopropyl group. Emamectin is a mixture, typically consisting of 10% B1b and 90% B1a.[7]
Avermectin biosynthesis is classified into three stages: the formation of the polyketide-derived initial aglycone, modification of the initial aglycone to produce avermectin aglycones, and glycosylation of avermectin aglycones to generate avermectins.[8]
Uses
Emamectin is widely used in controlling lepidopterous pests (order of insects that as larvae are caterpillars and as adults have four broad wings including butterflies, moths, and skippers) in agricultural products in the US, Japan, Canada, and recently Taiwan. The low-application rate of the active ingredient needed (~6 g/acre) and broad-spectrum applicability as an insecticide has gained emamectin significant popularity among farmers.[7]
Emamectin has been shown to possess a greater ability to reduce the colonization success of engraver beetles and associated wood borers in loblolly pines (Pinus taeda L).6 A 2006 study regarding bolt-injections of four types of pesticides found emamectin to be the greatest reducer against these species with respect to the amount of larval feeding, length, and number of egg galleries.6 Formation of long vertical lesions in the phloem and xylem surrounding emamectin injection points were found indicating some level of tree-toxicity to the emamectin.[9]
A water-soluble preparation of emamectin in polysorbate, acetone, and methanol was shown to prevent the wilting of Japanese black pine trees inoculated with pine-wood nematodes (Bursaphelenchus xylophilus).[2] Previous treatment of B. xylophilus infections involved eradicating the local population of Japanese pine sawyers associated with the spread of the nematode.
Emamectin has also been successfully employed by fish farmers in the control of sea lice in Atlantic salmon.[10][11] The United Kingdom, Chile, Ireland, Iceland, Finland, the Faroe Islands, Spain, and Norway are currently registered to use emamectin in their fish feed.[10] Removal of the afflicting sea louse represents an increase in the integrity of their salmonid product due to the subsequent reduction of bacterial and viral pathogens possibly carried by the sea lice. Emamectin has shown efficacy against all life-cycle stages of Lepeophtheirus salmonis (Salmon louse) and Caligus elongatus (Sea louse), preventing maturation to the reproductive stage.[11]
A related dihydroxy avermectin B1 compound, ivermectin, is utilized orally in humans as an acaricide and insecticide for the treatment of strongyloidiasis and onchocerciasis. Veterinarians also employ ivermectin in the treatment of heartworms in dogs and other infestations.[7]
Structure and properties
Emamectin, like other avermectins, is a hydrophobic 16-membered macrocyclic lactone.[2] Emamectin differs from avermectins B1a and B1b by the presence of a hydroxyl group at the 4”-epimethylamino group rather than the 4”-position. Avermectins are pentacyclic polyketide-derived compounds linked to a disaccharide of the methylated deoxysugar oleandrose.[8]
The determination of the active-site for avermectins is difficult due to poor solubility and lipophilicity of these compounds.
Toxicology and metabolism
Emamectin works as a chloride channel activator by binding gamma aminobutyric acid (GABA) receptor and glutamate-gated chloride channels disrupting nerve signals within arthropods.[6][12] The compound stimulates the release of GABA from the synapses between nerve cells and while additionally increasing GABA’s affinity for its receptor on the post-junction membrane of muscle cells in insects and arthropods.[11] The stronger binding of GABA increases the cells permeability to chloride ions within the cell due to the hypotonic concentration gradient.[11] Neurotransmission is thereby reduced by subsequent hyperpolarisation and the elimination of signal transduction.[11]
References
- ↑ US application 2009281175, Kaoukhov, A. & Cousin, C., "Avermectin Compounds and Treatment of Dermatological Disorders in Humans Therewith", published 2009-11-12, assigned to Galderma
- ↑ 2.0 2.1 2.2 US application 2010168043, Grossman,D.M. & Cox,D., "Method for the Protection of Trees", published 2010-07-01, assigned to Syngenta Crop Protection
- ↑ Waddy, S.; Merritt, V.; Hamilton-Gibson, M.; Aiken, D.; Burridge, L. (2007). "Relationship between dose of emamectin benzoate and molting response of ovigerous American lobsters (Homarus americanus)". Ecotoxicology and Environmental Safety 67 (1): 95–99. doi:10.1016/j.ecoenv.2006.05.002. PMID 16815547.
- ↑ US application 2011110906, Andersch, W.; Evans, P. & Springer, B. et al., "Combinations of biological control agents and insecticides or fungicides", published 2011-05-12, assigned to Bayer Cropscience
- ↑ Poland T.M. et al. (2010). "Management tactics for Emerald Ash Borer: Chemical and Biological Control" (PDF). General technical report GTR-NRS-P-75: 21st USDA Interagency Research Forum on Invasive Species. USDA.
- ↑ 6.0 6.1 Grant, A. N. (2002). "Medicines for sea lice". Pest Management Science 58 (6): 521–527. doi:10.1002/ps.481. PMID 12138618.
- ↑ 7.0 7.1 7.2 Yen, T. H.; Lin, J. L. (2004). "Acute poisoning with emamectin benzoate". Journal of toxicology. Clinical toxicology 42 (5): 657–661. doi:10.1081/clt-200026968. PMID 15462160.
- ↑ 8.0 8.1 McGonigle, I.; Lummis, S. C. R. (2010). "Molecular Characterization of Agonists That Bind to an Insect GABA Receptor". Biochemistry 49 (13): 2897–2902. doi:10.1021/bi901698c. PMC 2852148. PMID 20180551.
- ↑ Takai, K.; Soejima, T.; Suzuki, T.; Kawazu, K. (2001). "Development of a water-soluble preparation of emamectin benzoate and its preventative effect against the wilting of pot-grown pine trees inoculated with the pine wood nematode, Bursaphelenchus xylophilus". Pest Management Science 57 (5): 463–466. doi:10.1002/ps.301. PMID 11374165.
- ↑ 10.0 10.1 Ikeda, H.; Ōmura, S. (1997). "Avermectin Biosynthesis". Chemical Reviews 97 (7): 2591–2610. doi:10.1021/cr960023p. PMID 11851473.
- ↑ 11.0 11.1 11.2 11.3 11.4 Rodríguez, E. M.; Medesani, D. A.; Fingerman, M. (2007). "Endocrine disruption in crustaceans due to pollutants: A review". Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology 146 (4): 661–671. doi:10.1016/j.cbpa.2006.04.030. PMID 16753320.
- ↑ "Emamectin Benzoate" (PDF). SERA TR-052-23-03b Human Health and Ecological Risk Assessment. Atlanta GA: USDA/Forest Service. 2008. Retrieved 2011-10-30.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||


.png)