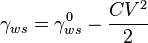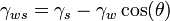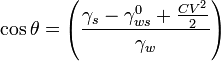Electrowetting
Electrowetting is the modification of the wetting properties of a surface (which is typically hydrophobic) with an applied electric field.
History
The electrowetting behavior of mercury and other liquids on variably charged surfaces was probably first explained by Gabriel Lippmann in 1875[1] and was certainly observed much earlier. A.N. Frumkin used surface charge to change the shape of water drops in 1936.[2] The term electrowetting was first introduced in 1981 by G.Beni and S.Hackwood to describe an effect proposed for designing a new type of display device for which they received a patent.[3] The use of a "fluid transistor" in microfluidic circuits for manipulating chemical and biological fluids was first investigated by J. Brown in 1980 and later funded in 1984-1988 under NSF Grants 8760730 & 8822197,[4] employing insulating dielectric and hydrophobic layer(s) (EWOD), immiscible fluids, DC or RF power; and mass arrays of miniature interleaved (saw tooth) electrodes with large or matching Indium tin oxide (ITO) electrodes to digitally relocate nano droplets in linear, circular and directed paths, pump or mix fluids, fill reservoirs and control fluid flow electronically or optically. Later, in collaboration with J. Silver at the NIH, EWOD-based electrowetting was disclosed for single and immiscible fluids to move, separate, hold and seal arrays of digital PCR sub-samples.[5]
Electrowetting using an insulating layer on top of the bare electrodes was later studied by Bruno Berge in 1993.[6] Electrowetting on this dielectric-coated surface is called electrowetting-on-dielectric (EWOD)[7] to distinguish it from the conventional electrowetting on the bare electrode. Microfluidic manipulation of liquids by electrowetting was demonstrated first with mercury droplets in water[8] and later with water in air[7] and water in oil.[9] Manipulation of droplets on a two-dimensional path was demonstrated later.[10][11] If the liquid is discretized and programmably manipulated, the approach is called "Digital Microfluidic Circuits"[12][13] or "Digital Microfluidics".[14] Discretization by electrowetting-on-dielectric (EWOD) was first demonstrated by Cho, Moon and Kim,[15] completing the four basic digital microfluidic functions of creating, transporting, dividing and merging droplets on chip by electrowetting
Since then, a large number of applications based on electrowetting have been demonstrated. Currently five companies are at the forefront in commercializing electrowetting-based applications based on Cytonix[16] and Berge's later research: Clinical diagnostics by Advanced Liquid Logic[17] which was spun out of Duke University, electronic paper by both Gamma Dynamics,[18] which was spun out of the University of Cincinnati, and Liquavista[19] which was spun out of Philips Research, liquid lenses by Varioptic,[20] and Digital PCR by Life Technologies and Sequenom. In some of these applications, electrowetting allows large numbers of droplets to be independently manipulated under direct electrical control without the use of external pumps, valves or even fixed channels. In e-paper and liquid lenses, droplets are manipulated in-place whereas in clinical diagnostics applications, droplets are moved around on the platform.
Electrowetting theory
.svg.png)
The electrowetting effect has been defined as "the change in solid-electrolyte contact angle due to an applied potential difference between the solid and the electrolyte". The phenomenon of electrowetting can be understood in terms of the forces that result from the applied electric field.[21][22] The fringing field at the corners of the electrolyte droplet tends to pull the droplet down onto the electrode, lowering the macroscopic contact angle and increasing the droplet contact area. Alternatively, electrowetting can be viewed from a thermodynamic perspective. Since the surface tension of an interface is defined as the Gibbs free energy required to create a certain area of that surface, it contains both chemical and electrical components, and charge becomes a significant term in that equation. The chemical component is just the natural surface tension of the solid/electrolyte interface with no electric field. The electrical component is the energy stored in the capacitor formed between the conductor and the electrolyte.
The simplest derivation of electrowetting behavior is given by considering its thermodynamic model. While it is possible to obtain a detailed numerical model of electrowetting by considering the precise shape of the electrical fringing field and how it affects the local droplet curvature, such solutions are mathematically and computationally complex. The thermodynamic derivation proceeds as follows. Defining the relevant surface tensions as:
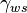 - The total, electrical and chemical, surface tension between the electrolyte and the conductor
- The total, electrical and chemical, surface tension between the electrolyte and the conductor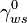 - The surface tension between the electrolyte and the conductor at zero electric field
- The surface tension between the electrolyte and the conductor at zero electric field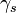 - The surface tension between the conductor and the external ambient
- The surface tension between the conductor and the external ambient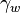 - The surface tension between the electrolyte and the external ambient
- The surface tension between the electrolyte and the external ambient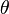 - The macroscopic contact angle between the electrolyte and the dielectric
- The macroscopic contact angle between the electrolyte and the dielectric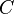 - The capacitance of the interface, єrє0/t, for a uniform dielectric of thickness t and permittivity єr
- The capacitance of the interface, єrє0/t, for a uniform dielectric of thickness t and permittivity єr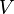 - The effective applied voltage, integral of the electric field from the electrolyte to the conductor
- The effective applied voltage, integral of the electric field from the electrolyte to the conductor
Relating the total surface tension to its chemical and electrical components gives:
The contact angle is given by the Young-Dupre equation, with the only complication being that the total surface energy 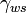 is used:
is used:
Combining the two equations gives the dependence of θ on the effective applied voltage as:
An additional complication is that liquids also exhibit a saturation phenomenon: after certain voltage, the saturation voltage, the further increase of voltage will not change the contact angle, and with extreme voltages the interface will only show instabilities.
However, surface charge is but one component of surface energy, and other components are certainly perturbed by induced charge. So, a complete explanation of electrowetting is unquantified, but it should not be surprising that these limits exist.
It was recently shown[23] that contact angle saturation can be explained if electrowetting is observed as a global phenomenon affected by the detailed geometry of the system. Within this framework it is predicted that reversed electrowetting is also possible (contact angle grows with the voltage).
It has also been experimentally shown by Chevaloitt [24] that contact angle saturation is invariant to all materials parameters, thus revealing that a universal theory for saturation is still lacking, and that when good materials are utilized, most saturation theories are invalid. This same paper further suggests that electrohydrodynamic instability may be the source of saturation, a theory that is unproven but being suggested by several other groups as well.
Reverse electrowetting
Reverse electrowetting[25] can be used to harvest energy via a mechanical-to-electrical engineering scheme.
Photoelectrowetting
Photoelectrowetting[26] can be observed if the conductor in the liquid/insulator/conductor stack used for electrowetting is replaced by a semiconductor. By optically modulating the number of carriers in space-charge region of the semiconductor, the contact angle of a liquid droplet can be altered in a continuous way. This effect can be explained by a modification of the Young-Lippmann equation.
Materials
For reasons that are still under investigation, only a limited set of surfaces exhibit the theoretically predicted electrowetting behavior. Amorphous fluoropolymers are by far the best electrowetting materials discovered so far, and it has been found that their behaviour can be enhanced by the appropriate patterning. Three types of such polymers are commercially available: FluoroPel hydrophobic and superhydrophobic V-series polymers are sold by Cytonix, CYTOP is sold by Asahi Glass Co., and Teflon AF is sold by DuPont.
Applications
Electrowetting is now used in a wide range of applications from modular to adjustable lenses, electronic displays (e-paper) and switches for optical fibers. Electrowetting has recently been evoked for manipulating Soft Matter particularly, suppressing coffee stain effect.[27] Furthermore, filters with Electrowetting functionality has been suggested for cleaning oil spills and separating oil-water mixtures.[28]
International meeting
An international meeting for electrowetting is held every two years. The most recent meeting was June 23rd to 25th, 2014, in Cincinnati, USA: http://secs.ceas.uc.edu/electrowetting2014/
The previous hosts of the electrowetting meeting are: Mons (1999), Eindhoven (2000), Grenoble (2002), Blaubeuren (2004), Rochester (2006), Los Angeles (2008), Pohang (2010), and Athens (2012).
See also
References
- ↑ M.G. Lippmann,"Relation entre les phénomènes électriques et capillaires." Ann. Chim. Phys, 5:494, 1875
- ↑ A. Frumkin, Об явлениях смачивания и прилипания пузырьков, I (On the phenomena of wetting and adhesion of the bubbles, I). Zhurnal Fizicheskoi Khimii (J Phys Chem USSR),12: 337- 345 (1938).
- ↑ G. Beni and S. Hackwood, Appl. Phys. Lett. 38, 4, pp.207-209, 1981
- ↑
- ↑
- ↑ B. Berge, "Électrocapillarité et mouillage de films isolants par l'eau", C.R. Acad. Sci. Paris, t. 317, Série II, p. 157-163, 1993.
- ↑ 7.0 7.1 J. Lee, "Microactuation by Continuous Electrowetting and Electrowetting: Theory, Fabrication, and Demonstration," PhD Thesis, University of California, Los Angeles, 2000
- ↑ J. Lee and C.-J. Kim, "Liquid Micromotor Driven by Continuous Electrowetting", Proc. IEEE Micro Electro Mechanical Systems Workshop, Heidelberg, Germany, Jan. 1998, pp. 538-543
- ↑ M.G. Pollack, R.B. Fair and A.D. Shenderov, "Electrowetting-based actuation of liquid droplets for microfluidic applications", Applied Physics Letters, vol. 77 (11), 2000
- ↑ S.-K. Fan, P.-P. de Guzman, and C.-J. Kim, "EWOD Driving of Droplet on NxM Grid Using Single-Layer Electrode Patterns, Tech. Dig., Solid-State Sensor, Actuator, and Microsystems Workshop, Hilton Head Island, SC, June 2002, pp. 134-137
- ↑ J. Gong and C.-J. Kim, "Two-Dimensional Digital Microfluidic System by Multi-Layer Printed Circuit Board", Proc. IEEE Conf. MEMS, Orlando, FL, Jan. 2005, pp. 726-729
- ↑ C.-J. Kim, "Integrated Digital Microfluidic Circuits Operated by Electrowetting-on-Dielectrics (EWOD) Principle", granted in 2000 by Defense Advanced Research Projects Agency (DARPA), award number N66001-0130-3664
- ↑ C.-J. Kim, "Micropumping by Electrowetting", Proceedings of the ASME International Mechanical Engineering Congress and Exposition, November 2001, New York, NY, IMECE2001/HTD-24200.
- ↑ M.G. Pollack, Electrowetting-Based Microactuation Of Droplets For Digital Microfluidics, PhD Thesis, Duke University, 2001.
- ↑ S. K. Cho, H. Moon, and C.-J Kim, "Creating, Transporting, Cutting, and Merging Liquid Droplets by Electrowetting-Based Actuation for Digital Microfluidic Circuits", J. Microelectromechanical Systems, Vol. 12, 2003, pp. 70-80
- ↑
- ↑ Advanced Liquid Logic
- ↑ Gamma Dynamics
- ↑ LiquaVista
- ↑ Varioptic
- ↑ Chang, H.C., Yeo, L. (2009). Electrokinetically Driven Microfluidics and Nanofluidics. Cambridge University Press.
- ↑ Kirby, B.J. (2010). Micro- and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices. Cambridge University Press. ISBN 978-0-521-11903-0.
- ↑ A Model of Electrowetting, Reversed Electrowetting and Contact Angle Saturation. Dan Klarman, David Andelman, Michael Urbakh
- ↑ Experimental Validation of the Invariance of Electrowetting Contact Angle Saturation
- ↑ T. Krupenkin and J.A.Taylor , Nature Comms. Rep. 2, 448, (2011).
- ↑ S. Arscott, Sci. Rep. 1, 184, (2011). Scientific Reports: Nature Publishing Group.
- ↑ H.Burak Eral, D.Mampallil, F.Mugele "Suppressing the coffee stain effect: how to control colloidal self-assembly in evaporating drops using electrowetting", Soft Matter, 2011, 7, 4954-4958, doi:10.1039/C1SM05183K
- ↑ H.Burak Eral, R.Ruiter, J.Ruiter, J.M.Oh, C.Semprebon, M.Brinkmann, F.Mugele, "Reversible morphological transitions of a drop on a fiber", Soft Matter, 2011, 7 (11), 5138 - 5143, doi:10.1039/C0SM01403F
External links
- Fan-TASY Lab at National Taiwan University
- Wheeler Digital Microfluidics Group at the University of Toronto
- Electrowetting at the University of Cincinnati.
- Digital Microfluidics at Duke University
- Physics of Complex Fluids at University of Twente
- Diagram explaining electrowetting
- Progress with electrowetting displays
- Electrowetting flexible display at UC NanoLab, University of Cincinnati
- Liquidvista Low Frequency Electrowetting 6.2-inch Display