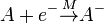Electron capture ionization
Electron capture ionization is the ionization of a gas phase atom or molecule by attachment of an electron to create an ion of the form A–•. The reaction is
where the M over the arrow denotes that to conserve energy and momentum a third body is required (the molecularity of the reaction is three).
Electron capture can be used in conjunction with chemical ionization.[1]
An electron capture detector is used in some gas chromatography systems.[2]
See also
Electron capture dissociation
References
- ↑ Donald F. Hunt; Frank W. Crow (1978), "Electron capture negative ion chemical ionization mass spectrometry", Analytical Chemistry 50 (13): 1781, doi:10.1021/ac50035a017
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "electron capture detector (in gas chromatography)".