Electrode potential
Electrode potential, E, in electrochemistry, according to an IUPAC definition,[1] is the electromotive force of a cell built of two electrodes:
- on the left-hand side is the standard hydrogen electrode, and
- on the right-hand side is the electrode the potential of which is being defined.
By convention:
- ECell = ECathode − EAnode
From the above, for the cell with the standard hydrogen electrode (potential of 0 by convention), one obtains:
- ECell = ERight − 0 = EElectrode
The left-right convention is consistent with the international agreement that redox potentials be given for reactions written in the form of reduction half-reactions.
Electrode potential is measured in volts (V).
Origin and interpretation
Electrode potential appears at the interface between an electrode and electrolyte due to the transfer of charged species across the interface, specific adsorption of ions at the interface, and specific adsorption/orientation of polar molecules, including those of the solvent.
Electrode potential is the electric potential on an electrode component. In a cell, there will be an electrode potential for the cathode, and an electrode potential for the anode. The difference between the two electrode potentials equals the cell potential.
ECell = ECathode − EAnode
The measured electrode potential may be either that at equilibrium on the working electrode ("reversible potential"), or a potential with a non-zero net reaction on the working electrode but zero net current ("corrosion potential", "mixed potential"), or a potential with a non-zero net current on the working electrode (like in galvanic corrosion or voltammetry). Reversible potentials can be sometimes converted to the standard electrode potential for a given electroactive species by extrapolation of the measured values to the standard state.
The value of the electrode potential under non-equilibrium depends on the nature and composition of the contacting phases, and on the kinetics of electrode reactions at the interface (see Butler-Volmer equation).
Measurement
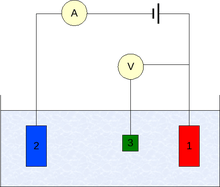
The measurement is generally conducted using a three-electrode setup (see the drawing):
- Working electrode
- Counter electrode
- Reference electrode (standard hydrogen electrode or an equivalent)
In case of non-zero net current on the electrode, it is essential to minimize the ohmic IR-drop in the electrolyte, e.g., by positioning the reference electrode near the surface of the working electrode (e.g., see Luggin capillary), or by using a supporting electrolyte of sufficiently high conductivity. The potential measurements are performed with the positive terminal of the electrometer connected to working electrode and the negative terminal to the reference electrode.
Potential difference of a cell assembled of two electrodes
Potential of a cell assembled of two electrodes can be determined from the two individual electrode potentials using:
ΔVcell = Ered,cathode - Ered,anode
or, equivalently,
ΔVcell = Ered,cathode + Eoxy,anode
This follows from the IUPAC definition of the electric potential difference of a galvanic cell,[2] according to which the electric potential difference of a cell is the difference of the potentials of the electrodes on the right and the left of the galvanic cell. When ΔVcell is positive, then positive electrical charge flows through the cell from the left electrode (anode) to the right electrode (cathode).
See also
- Standard electrode potential
- Absolute electrode potential
- Table of standard electrode potentials
- Electric potential
- Galvani potential
- Volta potential
- Potential difference (voltage)
- Overpotential
- Thermodynamic activity
- Nernst equation
References
- ↑ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006- ) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8. doi:10.1351/goldbook. Entry: "Electrode Potential"
- ↑ IUPAC Gold Book. Definition of the potential difference of a galvanic cell. http://goldbook.iupac.org/E01934.html