Electric double-layer capacitor

Electrical double-layer capacitors (EDLC) are, together with pseudocapacitors, part of a new type of electrochemical capacitors[1] called supercapacitors, also known as ultracapacitors. Supercapacitors do not have a conventional solid dielectric. The capacitance value of an electrochemical capacitor is determined by two storage principles:
- Double-layer capacitance – electrostatic storage of the electrical energy achieved by separation of charge in a Helmholtz double layer at the interface between the surface of a conductor electrode and an electrolytic solution electrolyte. The separation of charge distance in a double-layer is on the order of a few Ångströms (0.3–0.8 nm) and is static in origin.[2]
- Pseudocapacitance – Electrochemical storage of the electrical energy, achieved by redox reactions electrosorption or intercalation on the surface of the electrode by specifically adsorbed ions that results in a reversible faradaic charge-transfer on the electrode.[2]
Double-layer capacitance and pseudocapacitance both contribute to the total capacitance value of a supercapacitor.[3] However, the ratio of the two can vary greatly, depending on the design of the electrodes and the composition of the electrolyte. Pseudocapacitance can increase the capacitance value by as much as an order of magnitude over that of the double-layer by itself.[1]
Supercapacitors are divided into three families, based on the design of the electrodes:
- Double-layer capacitors – with carbon electrodes or derivatives with much higher static double-layer capacitance than the faradaic pseudocapacitance
- Pseudocapacitors – with electrodes made of metal oxides or conducting polymers with much higher faradaic pseudocapacitance than the static double-layer capacitance
- Hybrid capacitors – capacitors with special electrodes that exhibit both significant double-layer capacitance and pseudocapacitance, such as lithium-ion capacitors

Supercapacitors have the highest available capacitance values per unit volume and the greatest energy density of all capacitors. They can have capacitance values of 10,000 times that of electrolytic capacitors; up to 12,000 F at working voltages of 1.2 V.[1] Supercapacitors bridge the gap between capacitors and rechargeable batteries. In terms of specific energy, as well as in terms of specific power, this gap covers several orders of magnitude. However, batteries still have about ten times the capacity of supercapacitors.[4] While existing supercapacitors have energy densities that are approximately 10% of a conventional battery, their power density is generally 10 to 100 times as great. This makes charge and discharge cycles of supercapacitors much faster than batteries. Additionally, they will tolerate many more charge and discharge cycles than batteries.
In these electrochemical capacitors, the electrolyte is the conductive connection between the two active electrodes. This distinguishes them from electrolytic capacitors, in which the electrolyte is the cathode and thus forms the second electrode.
Supercapacitors are polarized and must operate with the correct polarity. Polarity is controlled by design with asymmetric electrodes, or, for symmetric electrodes, by a potential applied during manufacture.
Supercapacitors support a broad spectrum of applications for power and energy requirements, including:
- Long duration low current for memory back up in (SRAMs)
- Power electronics that require very short, high current, as in the KERS system in Formula 1 cars
- Recovery of braking energy in vehicles
Concept


In a conventional capacitor, energy is stored by moving charge carriers, typically electrons, from one metal plate to another. This charge separation creates a potential between the two plates, which can be harnessed in an external circuit. The total energy stored in this fashion increases with both the amount of charge stored and the potential between the plates. The amount of charge stored per unit voltage is essentially a function of the size, the distance and the material properties of the plates and the material in between the plates (the dielectric), while the potential between the plates is limited by the breakdown field strength of the dielectric. The dielectric controls the capacitor's voltage. Optimizing the material leads to higher energy density for a given size.
EDLCs do not have a conventional dielectric. Instead of two plates separated by an intervening insulator, these capacitors use virtual plates made of two layers of the same substrate. Their electrochemical properties, the so-called "electrical double layer", result in the effective separation of charge despite the vanishingly thin (on the order of nanometers) physical separation of the layers. The lack of need for a bulky layer of dielectric and the porosity of the material used, permits the packing of plates with much larger surface area into a given volume, resulting in high capacitances in small packages.
In an electrical double layer, each layer is quite conductive, but the physics at the interface between them means that no significant current can flow between the layers. The double layer can withstand only a low voltage, which means that higher voltages are achieved by matched series-connected individual EDLCs, much like series-connected cells in higher-voltage batteries.
EDLCs have much higher power density than batteries. Power density combines the energy density with the speed at which the energy can be delivered to the load. Batteries, which are based on the movement of charge carriers in a liquid electrolyte, have [5] relatively slow charge and discharge times. Capacitors can be charged or discharged at a rate that is typically limited by the heat tolerance of the electrodes.
While existing EDLCs have energy densities that are perhaps 1/10 that of a conventional battery, their power density is generally 10 to 100 times as great. This makes them most suited to an intermediary role between electrochemical batteries and electrostatic capacitors, where neither sustained energy release nor immediate power demands dominate.
History
Construction
- Styles of supercapacitors with activated carbon electrodes
-
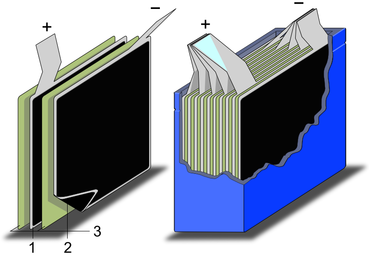
Schematic construction of a wound supercapacitor
1.Terminals, 2.Safety vent, 3.Sealing disc, 4.Aluminum can, 5.Positive pole, 6.Separator, 7.Carbon electrode, 8.Collector, 9.Carbon electrode, 10.Negative pole -
Schematic construction of a supercapacitor with stacked electrodes
1.Positive electrode, 2.Negative electrode,
3.Separator
Each EDLC cell consists of two electrodes, a separator and an electrolyte. The two electrodes are often electrically connected to their terminals via a metallic collector foil. The electrodes are usually made from activated carbon since this material is electrically conductive and has a very large surface area to increase the capacitance. The electrodes are separated by an ion permeable membrane (separator) used as an insulator to prevent short circuits between the electrodes. This composite is rolled or folded into a cylindrical or rectangular shape and can be stacked in an aluminium can or a rectangular housing. The cell is typically impregnated with a liquid or viscous electrolyte, either organic or aqueous, although some are solid state. The electrolyte depends on the application, the power requirement or peak current demand, the operating voltage and the allowable temperature range. The outer housing is hermetically sealed.
Comparisons
Advantages of supercapacitors include:
- Long life, with little degradation over hundreds of thousands of charge cycles. Due to the capacitor's high number of charge-discharge cycles (compared to 200 to 1000 for most rechargeable batteries) it will last for the entire lifetime of most devices, which makes the device environmentally friendly. Rechargeable batteries wear out typically over a few years and their highly reactive chemical electrolytes present a disposal and safety hazard. Battery lifetime can be optimised by charging only under favorable conditions, at an ideal rate and for some chemistries, as infrequently as possible. EDLCs can help in conjunction with batteries by acting as a charge conditioner, storing energy from other sources for load balancing purposes and then using any excess energy to optimally charge batteries.
- Low cost per cycle
- Good reversibility
- Fast charge and discharge.
- Low internal resistance—Low ESR and consequent high cycle efficiency (95% or more)
- Low heating levels during charge and discharge
- High output power
- High specific power/power density—According to the Institute of Transportation Studies, the specific power of electric double-layer capacitors can exceed 6 kW/kg at 95% efficiency.[6]
- Improved safety—Uses non-corrosive electrolytes and low material toxicity.
- Simple charge methods—no danger of overcharging, thus no need for full-charge detection.
- In conjunction with rechargeable batteries, some applications use EDLC to supply energy directly, reducing battery cycling and extending life.
Disadvantages include:
- Low energy density—The amount of energy stored per unit weight is generally lower than that of electrochemical batteries (3 to 5 W·h/kg, although 85 W·h/kg has been achieved in the lab[7] as of 2010 compared to 30 to 40 W·h/kg for a lead acid battery, 100 to 250 W·h/kg for a lithium-ion battery and about 0.1% of the volumetric energy density of gasoline).
- High dielectric absorption—highest of any type of capacitor.
- High self-discharge—the rate is considerably higher than that of an electrochemical battery.
- Low maximum voltage—series connections are needed to obtain higher voltages and voltage balancing may be required.
- Rapid voltage drop—Unlike batteries, the voltage across any capacitor drops significantly as it discharges. Effective energy recovery requires complex electronic control and switching equipment, with consequent energy loss.
- Spark hazard—Low internal resistance allows extremely rapid discharge when shorted, resulting in a spark hazard generally much greater than with batteries.
Materials

In general, EDLCs improve storage density through the use of a nanoporous material, typically activated charcoal, in place of the conventional insulating dielectric barrier. Activated charcoal is an extremely porous, "spongy" form of carbon with an extraordinarily high specific surface area—a common approximation is that 1 gram (a pencil-eraser-sized amount) has a surface area of roughly 250 square metres (2,700 sq ft)—about the size of a tennis court. It is typically a powder made up of extremely fine but very "rough" particles, which, in bulk, form a low-density heap with many holes. As the surface area of such a material is many times greater than a traditional material like aluminum, many more charge carriers (ions or radicals from the electrolyte) can be stored in a given volume. As carbon is not a good insulator (vs. the excellent insulators used in conventional devices), in general EDLCs are limited to low potentials on the order of 2 to 3 V and thus are "stacked" (connected in series) to supply higher voltages.
Activated charcoal is not the "perfect" material for this application. The charge carriers it provides are far larger than the holes left in the charcoal, which are too small to accept them, limiting the storage. The mismatch is exacerbated when the carbon is surrounded by solvent molecules.
As of 2010 virtually all commercial supercapacitors use powdered activated carbon made from coconut shells.[8] Higher performance devices are available, at a significant cost increase, based on synthetic carbon precursors that are activated with potassium hydroxide (KOH).
Research materials
Properties
Applications
Market
See also
- Electric vehicle battery
- Types of capacitors
- Nanoflower
- Rechargeable electricity storage system
- Flywheel energy storage
- List of emerging technologies
- Lithium ion capacitor
- Self-powered equipment
- Mechanically powered flashlight
- Conjugated microporous polymer
References
- ↑ 1.0 1.1 1.2 B. E. Conway (1999) (in German), Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, Berlin: Springer, pp. 1–8, ISBN 0306457369, //books.google.com/books?id=8yvzlr9TqI0C&pg=PA1 See also Brian E. Conway in Electrochemistry Encyclopedia: Electrochemical Capacitors — Their Nature, Function and Applications
- ↑ 2.0 2.1 Adam Marcus Namisnyk. (PDF) (in German) http://services.eng.uts.edu.au/cempe/subjects_JGZ/eet/Capstone%20thesis_AN.pdf. Retrieved 2011-06-24. Missing or empty
|title=(help) - ↑ Elzbieta Frackowiak, Francois Beguin, PERGAMON, Carbon 39 (2001) 937–950, Carbon materials for the electrochemical storage of energy in Capacitors PDF
- ↑ Kötz, R.; Carlen, M. (2000). "Principles and applications of electrochemical capacitors" (PDF). Electrochimica Acta 45: 2483–2498. doi:10.1016/s0013-4686(00)00354-6.
- ↑ Garthwaite, Josie (12 July 2011). "How ultracapacitors work (and why they fall short)". Earth2Tech. GigaOM Network. Retrieved 13 July 2011.
- ↑ Prototype Test APowerCap press release: Results highly appreciated by Ultracapacitor Experts, 2006.
- ↑
- ↑ Laine, Jorge; Simon Yunes (1992). "Effect of the preparation method on the pore size distribution of activated carbon from coconut shell". Carbon 30 (4): 601–604. doi:10.1016/0008-6223(92)90178-Y.
External links
- Super Capacitor Seminar
- Article on ultracapacitors at electronicdesign.com
- A new version of an old idea is threatening the battery industry (The Economist).
- An Encyclopedia Article From the Yeager center at CWRU.
- Ultracapacitors & Supercapacitors Forum
- Special Issue of Interface magazine on electrochemical capacitors
- Nanoflowers Improve Ultracapacitors: A novel design could boost energy storage (Technology Review) and Can nanoscopic meadows drive electric cars forward? (New Scientist)
- If the cap fits... How supercapacitors can help to solve power problems in portable products.
- A web that describes the development of solid-state and hybrid supercapacitors from CNR-ITAE (Messina) Italy