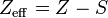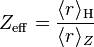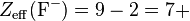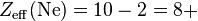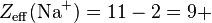Effective nuclear charge
The effective nuclear charge (often symbolized as 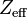 or
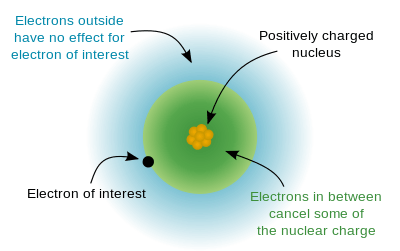
or 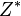 ) is the net positive charge experienced by an electron in a multi-electron atom. The term "effective" is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge by the repelling effect of inner-layer electrons. The effective nuclear charge experienced by the outer shell electron is also called the core charge. It is possible to determine the strength of the nuclear charge by the oxidation number of the atom.
) is the net positive charge experienced by an electron in a multi-electron atom. The term "effective" is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge by the repelling effect of inner-layer electrons. The effective nuclear charge experienced by the outer shell electron is also called the core charge. It is possible to determine the strength of the nuclear charge by the oxidation number of the atom.
Calculation
In an atom with one electron, that electron experiences the full charge of the positive nucleus. In this case, the effective nuclear charge can be calculated from Coulomb's law.
However, in an atom with many electrons the outer electrons are simultaneously attracted to the positive nucleus and repelled by the negatively charged electrons. The effective nuclear charge on such an electron is given by the following equation:
where
- Z is the number of protons in the nucleus (atomic number), and
- S is the average number of electrons between the nucleus and the electron in question (the number of nonvalence electrons).
S can be found by the systematic application of various rule sets, the simplest of which is known as "Slater's rules" (named after John C. Slater). Douglas Hartree defined the effective Z of a Hartree–Fock orbital to be:
where
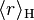 is the mean radius of the orbital for hydrogen, and
is the mean radius of the orbital for hydrogen, and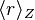 is the mean radius of the orbital for an electron configuration with nuclear charge Z.
is the mean radius of the orbital for an electron configuration with nuclear charge Z.
Example
Consider a sodium cation, a fluorine anion, and a neutral neon atom. Each has 10 electrons, and the number of nonvalence electrons is 2 (10 total electrons - 8 valence) but the effective nuclear charge varies because each has a different atomic number:
So the sodium cation has the largest effective nuclear charge, and thus the smallest radius.
Values
Updated values of screening constants were provided by Clementi et al.[1][2]
| H | He | |||||||||||||||||
| Z | 1 | 2 | ||||||||||||||||
| 1s | 1.000 | 1.688 | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | |||||||||||
| Z | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||||||
| 1s | 2.691 | 3.685 | 4.680 | 5.673 | 6.665 | 7.658 | 8.650 | 9.642 | ||||||||||
| 2s | 1.279 | 1.912 | 2.576 | 3.217 | 3.847 | 4.492 | 5.128 | 5.758 | ||||||||||
| 2p | 2.421 | 3.136 | 3.834 | 4.453 | 5.100 | 5.758 | ||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| Z | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||
| 1s | 10.626 | 11.609 | 12.591 | 13.575 | 14.558 | 15.541 | 16.524 | 17.508 | ||||||||||
| 2s | 6.571 | 7.392 | 8.214 | 9.020 | 9.825 | 10.629 | 11.430 | 12.230 | ||||||||||
| 2p | 6.802 | 7.826 | 8.963 | 9.945 | 10.961 | 11.977 | 12.993 | 14.008 | ||||||||||
| 3s | 2.507 | 3.308 | 4.117 | 4.903 | 5.642 | 6.367 | 7.068 | 7.757 | ||||||||||
| 3p | 4.066 | 4.285 | 4.886 | 5.482 | 6.116 | 6.764 | ||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| Z | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 |
| 1s | 18.490 | 19.473 | 20.457 | 21.441 | 22.426 | 23.414 | 24.396 | 25.381 | 26.367 | 27.353 | 28.339 | 29.325 | 30.309 | 31.294 | 32.278 | 33.262 | 34.247 | 35.232 |
| 2s | 13.006 | 13.776 | 14.574 | 15.377 | 16.181 | 16.984 | 17.794 | 18.599 | 19.405 | 20.213 | 21.020 | 21.828 | 22.599 | 23.365 | 24.127 | 24.888 | 25.643 | 26.398 |
| 2p | 15.027 | 16.041 | 17.055 | 18.065 | 19.073 | 20.075 | 21.084 | 22.089 | 23.092 | 24.095 | 25.097 | 26.098 | 27.091 | 28.082 | 29.074 | 30.065 | 31.056 | 26.047 |
| 3s | 8.680 | 9.602 | 10.340 | 11.033 | 11.709 | 12.368 | 13.018 | 13.676 | 14.322 | 14.961 | 15.594 | 16.219 | 16.996 | 17.790 | 18.596 | 19.403 | 20.219 | 21.033 |
| 3p | 7.726 | 8.658 | 9.406 | 10.104 | 10.785 | 11.466 | 12.109 | 12.778 | 13.435 | 14.085 | 14.731 | 15.369 | 16.204 | 17.014 | 17.850 | 18.705 | 19.571 | 20.434 |
| 4s | 3.495 | 4.398 | 4.632 | 4.817 | 4.981 | 5.133 | 5.283 | 5.434 | 5.576 | 5.711 | 5.842 | 5.965 | 7.067 | 8.044 | 8.944 | 9.758 | 10.553 | 11.316 |
| 3d | 7.120 | 8.141 | 8.983 | 9.757 | 10.528 | 11.180 | 11.855 | 12.530 | 13.201 | 13.878 | 15.093 | 16.251 | 17.378 | 18.477 | 19.559 | 20.626 | ||
| 4p | 6.222 | 6.780 | 7.449 | 8.287 | 9.028 | 9.338 | ||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Z | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 |
| 1s | 36.208 | 37.191 | 38.176 | 39.159 | 40.142 | 41.126 | 42.109 | 43.092 | 44.076 | 45.059 | 46.042 | 47.026 | 48.010 | 48.992 | 49.974 | 50.957 | 51.939 | 52.922 |
| 2s | 27.157 | 27.902 | 28.622 | 29.374 | 30.125 | 30.877 | 31.628 | 32.380 | 33.155 | 33.883 | 34.634 | 35.386 | 36.124 | 36.859 | 37.595 | 38.331 | 39.067 | 39.803 |
| 2p | 33.039 | 34.030 | 35.003 | 35.993 | 36.982 | 37.972 | 38.941 | 39.951 | 40.940 | 41.930 | 42.919 | 43.909 | 44.898 | 45.885 | 46.873 | 47.860 | 48.847 | 49.835 |
| 3s | 21.843 | 22.664 | 23.552 | 24.362 | 25.172 | 25.982 | 26.792 | 27.601 | 28.439 | 29.221 | 30.031 | 30.841 | 31.631 | 32.420 | 33.209 | 33.998 | 34.787 | 35.576 |
| 3p | 21.303 | 22.168 | 23.093 | 23.846 | 24.616 | 25.474 | 26.384 | 27.221 | 28.154 | 29.020 | 29.809 | 30.692 | 31.521 | 32.353 | 33.184 | 34.009 | 34.841 | 35.668 |
| 4s | 12.388 | 13.444 | 14.264 | 14.902 | 15.283 | 16.096 | 17.198 | 17.656 | 18.582 | 18.986 | 19.865 | 20.869 | 21.761 | 22.658 | 23.544 | 24.408 | 25.297 | 26.173 |
| 3d | 21.679 | 22.726 | 25.397 | 25.567 | 26.247 | 27.228 | 28.353 | 29.359 | 30.405 | 31.451 | 32.540 | 33.607 | 34.678 | 35.742 | 36.800 | 37.839 | 38.901 | 39.947 |
| 4p | 10.881 | 11.932 | 12.746 | 13.460 | 14.084 | 14.977 | 15.811 | 16.435 | 17.140 | 17.723 | 18.562 | 19.411 | 20.369 | 21.265 | 22.181 | 23.122 | 24.030 | 24.957 |
| 5s | 4.985 | 6.071 | 6.256 | 6.446 | 5.921 | 6.106 | 7.227 | 6.485 | 6.640 | (empty) | 6.756 | 8.192 | 9.512 | 10.629 | 11.617 | 12.538 | 13.404 | 14.218 |
| 4d | 15.958 | 13.072 | 11.238 | 11.392 | 12.882 | 12.813 | 13.442 | 13.618 | 14.763 | 15.877 | 16.942 | 17.970 | 18.974 | 19.960 | 20.934 | 21.893 | ||
| 5p | 8.470 | 9.102 | 9.995 | 10.809 | 11.612 | 12.425 | ||||||||||||
See also
- Slater-type orbitals
- Electronegativity
References
- ↑ Clementi, E.; Raimondi, D. L. (1963). "Atomic Screening Constants from SCF Functions". J. Chem. Phys 38 (11): 2686–2689. Bibcode:1963JChPh..38.2686C. doi:10.1063/1.1733573.
- ↑ Clementi, E.; Raimondi, D. L.; Reinhardt, W. P. (1967). "Atomic Screening Constants from SCF Functions. II. Atoms with 37 to 86 Electrons". Journal of Chemical Physics 47: 1300–1307. Bibcode:1967JChPh..47.1300C. doi:10.1063/1.1712084.
Resources
- Brown, Theodore; LeMay, H.E.; & Bursten, Bruce (2002). Chemistry: The Central Science (8th revised edition). Upper Saddle River, New Jersey 07458: Prentice-Hall. ISBN 0-13-061142-5.