Dunham expansion
In quantum chemistry, the Dunham expansion is an expression for the rotational-vibrational energy levels of a diatomic molecule: [1]
where v and J are the vibrational and rotational quantum numbers.
The constant coefficients 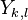 are called Dunham parameters with
are called Dunham parameters with 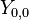 representing the electronic energy. The expression derives from a semiclassical treatment of a perturbational approach to deriving the energy levels.[2] The Dunham parameters are typically calculated by a least-squares fitting procedure of energy levels with the quantum numbers.
representing the electronic energy. The expression derives from a semiclassical treatment of a perturbational approach to deriving the energy levels.[2] The Dunham parameters are typically calculated by a least-squares fitting procedure of energy levels with the quantum numbers.
Relation to conventional band spectrum constants
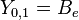 |
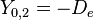 |
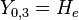 |
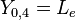 | |
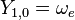 |
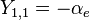 |
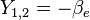 | ||
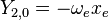 |
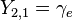 | |||
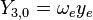 | ||||
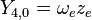 |
This table adapts the sign conventions from the book of Huber and Herzberg. [3]
See also
- Rotational-vibrational spectroscopy
References
- ↑ Dunham, J. L. (1932). "The Energy Levels of a Rotating Vibrator". Phys.Rev. 41: 721–731. doi:10.1103/PhysRev.41.721.
- ↑ Inostroza, N.; J.R. Letelier and M.L. Senent (2010). "On the numerical determination of Dunham’s coefficients: An application to X1 R + HCl isotopomers". Journal of Molecular Structure: THEOCHEM 947: 40–44. doi:10.1016/j.theochem.2010.01.037.
- ↑ Huber, K.P.; Herzberg, G. (1979). Molecular Spectra and Molecular Structure IV. Constants of diatomic molecules. New York: van Nostrand. ISBN 0-442-23394-9.
![E(v,J) = \sum_{k,l} Y_{k,l} (v+1/2)^k [J(J+1)]^l,](../I/m/2c83a368385feb9768bf1af3f931cdf4.png)