Dithiolane
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dithiolane | |||
| Other names
1,2-dithiolane, 1,3-dithiolane | |||
| Identifiers | |||
| ChEBI | CHEBI:38226 | ||
| ChemSpider | 71377 | ||
| |||
| Jmol-3D images | Image | ||
| PubChem | 79045 | ||
| |||
| Properties | |||
| C3H6S2 | |||
| Related compounds | |||
| Related compounds |
1,2-Ethanedithiol | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
A dithiolane is a sulfur heterocycle derived from cyclopentane by replacing two methylene bridges (-CH
2- units) with thioether groups. The parent compounds are 1,2-dithiolane and 1,3-dithiolane.
1,2-Dithiolanes, such as lipoic acid, are cyclic disulfides.
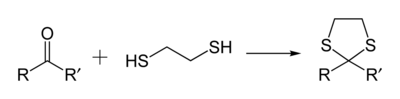
1,3-Dithiolanes are important as protecting groups for carbonyl compounds, since they are inert to a wide range of conditions. Reacting a carbonyl group with 1,2-ethanedithiol converts it to a 1,3-dithiolane, as detailed below.