Dipicolylamine
 | |
| Names | |
|---|---|
| Other names
Di-(2-picolyl)amine, DPA | |
| Identifiers | |
| 1539-42-0 | |
| Properties | |
| C12H13N3 | |
| Molar mass | 199.25 |
| Appearance | white solid |
| Density | 1.107 g/cm3 |
| Melting point | 139-141 °C |
| low | |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
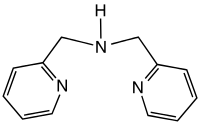
Dipicolylamine is an organic compound with the formula HN(CH2C5H4N)2. It is a white solid that is soluble in polar organic solvents. The molecule is a secondary amine with two picolyl substituents. The compound is a common tridentate ligand in coordination chemistry.[1][2]
The compound can be prepared by many methods, alkylation of picolinylamine with picolinyl chloride, deamination of picolinylamine, and reductive amination of picolinyl amine and pyridine-2-carboxaldehyde.
Related compounds
References
- ↑ Sakamoto, Takashi; Ojida, Akio; Hamachi, Itaru"Molecular recognition, fluorescence sensing, and biological assay of phosphate anion derivatives using artificial Zn(II)-Dpa complexes" Chemical Communications 2009, pp.141-152. doi:10.1039/B812374H
- ↑ Huy Tien Ngo, Xuejian Liu, Katrina A. Jolliffe "Anion recognition and sensing with Zn(II)–dipicolylamine complexes" Chem. Soc. Rev., 2012,41, 4928-4965. doi:10.1039/C2CS35087D