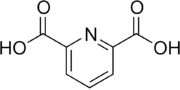
Dipicolinic acid
 | |
| Names | |
|---|---|
| IUPAC name
Pyridine-2,6-dicarboxylic acid | |
| Other names
2,6-Pyridinedicarboxylic acid | |
| Identifiers | |
| 499-83-2 | |
| ChEBI | CHEBI:46837 |
| ChEMBL | ChEMBL284104 |
| ChemSpider | 9940 |
| DrugBank | DB04267 |
| |
| Jmol-3D images | Image |
| PubChem | 10367 |
| |
| Properties | |
| Molecular formula |
C7H5NO4 |
| Molar mass | 167.12 g·mol−1 |
| Melting point | 248 °C (478 °F; 521 K) |
| Hazards | |
| Main hazards | Irritant (Xi) |
| R-phrases | R36/37/38 |
| S-phrases | S26 S36 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Dipicolinic acid (pyridine-2,6-dicarboxylic acid or PDC and DPA) is a chemical compound which composes 5% to 15% of the dry weight of bacterial spores.[2][3] It is implicated as responsible for the heat resistance of the endospore.[2][4]
However, mutants resistant to heat but lacking dipicolinic acid have been isolated, suggesting other mechanisms contributing to heat resistance are at work.[5]
Dipicolinic acid forms a complex with calcium ions within the endospore core. This complex binds free water molecules, causing dehydration of the spore. As a result, the heat resistance of macromolecules within the core increases. The calcium-dipicolinic acid complex also functions to protect DNA from heat denaturation by inserting itself between the nucleobases, thereby increasing the stability of DNA.[6]
Two genera of bacteria are known to produce endospores: the aerobic Bacillus and anaerobic Clostridium.[7]
Adrian Ponce, a chemist at the Jet Propulsion Laboratory has developed a technique for the detection of germinable endospores by staining dipicolinic acid with terbium. When the area is illuminated under ultraviolet light, live endospores glow in the presence of the terbium. The technique was developed for the validation of Spacecraft cleanliness, but it promises applications for the assessment of sterility more generally. The technique has the advantage of much greater speed over culture-based methods of endospore detection.[8][9]
It is also used to prepare dipicolinato ligated lanthanide and transition metal complexes for ion chromatography.[10]
References
- ↑ 2,6-Pyridinedicarboxylic acid at Sigma-Aldrich
- ↑ 2.0 2.1 Sliemandagger, TA.; Nicholson, WL. (2001). "Role of Dipicolinic Acid in Survival of Bacillus subtilis Spores Exposed to Artificial and Solar UV Radiation". Applied and Environmental Microbiology 67 (3): 1274–1279. doi:10.1128/aem.67.3.1274-1279.2001.
- ↑ Sci-Tech Dictionary. McGraw-Hill Dictionary of Scientific and Technical Terms, McGraw-Hill Companies, Inc.
- ↑ Madigan, M., J Martinko, J. Parker (2003). Brock Biology of Microorganisms, 10th edition. Pearson Education, Inc., ISBN 981-247-118-9.
- ↑ Prescott, L. (1993). Microbiology, Wm. C. Brown Publishers, ISBN 0-697-01372-3.
- ↑ Madigan. M, Martinko. J, Bender. K, Buckley. D, Stahl. D, (2014), Brock Biology of Microorganisms, 14th Edition, p. 78, Pearson Education Inc., ISBN 978-0-321-89739-8.
- ↑ Gladwin, M. (2008). Clinical Microbiology Made Ridiculously Simple, MedMaster, Inc., ISBN 0-940780-81-X.
- ↑ http://www.astrobio.net/topic/exploration/missions/spotting-spores/''. Missing or empty
|title=(help); - ↑ Yung, Pun To; Ponce, Adrian (Dec 2008). "Fast Sterility Assessment by Germinable-Endospore Biodosimetry". Appl Environ Microbiol. 74 (24): 7669–7674. doi:10.1128/AEM.01437-08. PMC 2607155. PMID 18836020.
- ↑ 2,6-Pyridinedicarboxylic acid at Sigma-Aldrich
External links
- JPL Develops High-Speed Test to Improve Pathogen Decontamination at JPL.
- Spotting Spores at Astrobiology Magazine.