Diphtheria toxin
| Diphtheria toxin, C domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 complex of diphtheria toxin and heparin-binding epidermal growth factor | |||||||||
| Identifiers | |||||||||
| Symbol | Diphtheria_C | ||||||||
| Pfam | PF02763 | ||||||||
| Pfam clan | CL0084 | ||||||||
| InterPro | IPR022406 | ||||||||
| SCOP | 1ddt | ||||||||
| SUPERFAMILY | 1ddt | ||||||||
| TCDB | 1.C.7 | ||||||||
| |||||||||
| Diphtheria toxin, T domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 complex of diphtheria toxin and heparin-binding epidermal growth factor | |||||||||
| Identifiers | |||||||||
| Symbol | Diphtheria_T | ||||||||
| Pfam | PF02764 | ||||||||
| InterPro | IPR022405 | ||||||||
| SCOP | 1ddt | ||||||||
| SUPERFAMILY | 1ddt | ||||||||
| TCDB | 1.C.7 | ||||||||
| |||||||||
| Diphtheria toxin, R domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 complex of diphtheria toxin and heparin-binding epidermal growth factor | |||||||||
| Identifiers | |||||||||
| Symbol | Diphtheria_R | ||||||||
| Pfam | PF01324 | ||||||||
| InterPro | IPR022404 | ||||||||
| SCOP | 1ddt | ||||||||
| SUPERFAMILY | 1ddt | ||||||||
| TCDB | 1.C.7 | ||||||||
| |||||||||
| tox diphtheria toxin precursor | |
|---|---|
| Identifiers | |
| Organism | |
| Symbol | tox |
| Entrez | 2650491 |
| RefSeq (Prot) | NP_938615 |
| UniProt | Q6NK15 |
| Other data | |
| EC number | 2.4.2.36 |
| Chromosome | genome: 0.19 - 0.19 Mb |
Diphtheria toxin is an exotoxin secreted by Corynebacterium diphtheriae, the pathogen bacterium that causes diphtheria. Unusually, the toxin gene is encoded by a bacteriophage (a virus that infects bacteria).[1] The toxin causes the disease diphtheria in humans by gaining entry into the cell cytoplasm and inhibiting protein synthesis.[2]
Structure
Diphtheria toxin is a single polypeptide chain of 535 amino acids consisting of two subunits linked by disulfide bridges, known as an A-B toxin. Binding to the cell surface of the B subunit (the less stable of the two subunits) allows the A subunit (the more stable part of the protein) to penetrate the host cell.[3]
The crystal structure of the diphtheria toxin homodimer has been determined to 2.5A resolution. The structure reveals a Y-shaped molecule consisting of 3 domains. Fragment A contains the catalytic C domain, and fragment B consists of the T and R domains[4][4]
- The N-terminal catalytic domain, known as the C domain, has an unusual beta+alpha fold.[5] The C domain blocks protein synthesis by transfer of ADP-ribose from NAD to a diphthamide residue of EF-2.[6][7]
- A central translocation domain, known as the T domain or TM domain. The T domain has a multi-helical globin-like fold with two additional helices at N-termini, but which has no counterpart to the first globin helix. This domain is thought to unfold in the membrane.[8] pH-induced conformational change in the T domain triggers insertion into the endosomal membrane and facilitates the transfer of the C domain into the cytoplasm.[6][7]
- A C-terminal receptor-binding domain, known as the R domain. This domain has a beta-sandwich fold consisting of nine strands in two sheets with Greek-key topology; it is a subclass of immunoglobin-like fold.[5] The R domain binds to cell surface receptor, permitting the toxin to enter the cell by receptor mediated endocytosis.[6][7]
Mechanism
The diphtheria toxin has the same mechanism of action as the enzyme NAD(+)—diphthamide ADP-ribosyltransferase (EC 2.4.2.36). It catalyzes the transfer of NAD+ to a diphthamide residue in eukaryotic elongation factor-2 (eEF2), inactivating this protein. It does so by ADP-ribosylating the unusual amino acid diphthamide. In this way, it acts as a RNA translational inhibitor. The catalysed reaction is as follows:
- NAD+ + peptide diphthamide
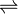 nicotinamide + peptide N-(ADP-D-ribosyl)diphthamide.
nicotinamide + peptide N-(ADP-D-ribosyl)diphthamide.
Diphtheria toxin has also been associated with the development of myocarditis. Myocarditis secondary to diphtheria toxin is considered one of the biggest risks to non-immunized children.
The exotoxin A of Pseudomonas aeruginosa uses a similar mechanism of action.
Lethal dose
Diphtheria toxin is extraordinarily potent.[3] The lethal dose for humans is about 0.1 μg of toxin per kg of bodyweight. A massive release of toxin into the body will likely cause lethal necrosis of the heart and liver.[9]
History
Diphtheria toxin was discovered in 1890 by Emil Adolf von Behring. In 1951, Freeman found that the toxin gene was not encoded on the bacterial chromosome, but by a lysogenic phage infecting all toxigenic strains.[10][11][12]
Clinical use
The drug denileukin diftitox uses diphtheria toxin as an antineoplastic agent. Resimmune™ is an immunotoxin which is in Clinical Trials in Cutaneous T cell lymphoma patients. It uses diphtheria toxin (truncated by the cell binding domain) coupled to anti-CD3ε Ab (UCHT1).
References
- ↑ TABLE 1. Bacterial virulence properties altered by bacteriophages from Patrick L. Wagner, Matthew K. Waldor (August 2002). "Bacteriophage Control of Bacterial Virulence". Infection and Immunity 70 (8): 3985–3993. doi:10.1128/IAI.70.8.3985-3993.2002. PMC 128183. PMID 12117903.
- ↑ Bell CE, Eisenberg D (1996). "Crystal structure of diphtheria toxin bound to nicotinamide adenine dinucleotide". Biochemistry 35 (4): 1137–1149. doi:10.1021/bi9520848. PMID 8573568.
- ↑ 3.0 3.1 Murphy JR (1996). "Corynebacterium Diphtheriae: Diphtheria Toxin Production". In Baron S et al. Medical microbiology (4 ed.). Galveston, Texas: Univ. of Texas Medical Branch. ISBN 0-9631172-1-1.
- ↑ 4.0 4.1 Choe S, Bennett MJ, Fujii G, Curmi PM, Kantardjieff KA, Collier RJ, Eisenberg D (May 1992). "The crystal structure of diphtheria toxin". Nature 357 (6375): 216–22. doi:10.1038/357216a0. PMID 1589020.
- ↑ 5.0 5.1 Bell CE, Eisenberg D (January 1997). "Crystal structure of nucleotide-free diphtheria toxin". Biochemistry 36 (3): 481–8. doi:10.1021/bi962214s. PMID 9012663.
- ↑ 6.0 6.1 6.2 Bennett MJ, Eisenberg D (September 1994). "Refined structure of monomeric diphtheria toxin at 2.3 A resolution". Protein Sci. 3 (9): 1464–75. doi:10.1002/pro.5560030912. PMC 2142954. PMID 7833808.
- ↑ 7.0 7.1 7.2 Bell CE, Eisenberg D (January 1996). "Crystal structure of diphtheria toxin bound to nicotinamide adenine dinucleotide". Biochemistry 35 (4): 1137–49. doi:10.1021/bi9520848. PMID 8573568.
- ↑ Bennett MJ, Choe S, Eisenberg D (September 1994). "Refined structure of dimeric diphtheria toxin at 2.0 A resolution". Protein Sci. 3 (9): 1444–63. doi:10.1002/pro.5560030911. PMC 2142933. PMID 7833807.
- ↑ Pappenheimer A (1977). "Diphtheria toxin.". Annu Rev Biochem 46 (1): 69–94. doi:10.1146/annurev.bi.46.070177.000441. PMID 20040.
- ↑ Freeman VJ (June 1951). "Studies on the virulence of bacteriophage-infected strains of Corynebacterium diphtheriae". J. Bacteriol. 61 (6): 675–88. PMC 386063. PMID 14850426.
- ↑ Freeman VJ, Morse IU (March 1952). "Further observations on the change to virulence of bacteriophage-infected avirulent strains of Corynebacterium diphtheria". J. Bacteriol. 63 (3): 407–14. PMC 169283. PMID 14927573.
- ↑ Diphtheria from Todar's Online Textbook of Bacteriology, Kenneth Todar 2009. Accessed 08 September 2010.
External links
- Diphtheria Toxin at the US National Library of Medicine Medical Subject Headings (MeSH)
- How Diphtheria Toxin Works - Animation
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
This article incorporates text from the public domain Pfam and InterPro IPR022406
This article incorporates text from the public domain Pfam and InterPro IPR022405
This article incorporates text from the public domain Pfam and InterPro IPR022404
