Diketene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
4-methylideneoxetan-2-one | |||
| Other names
γ-methylenebutyrolactone | |||
| Identifiers | |||
| 674-82-8 | |||
| ChemSpider | 12140 | ||
| |||
| Jmol-3D images | Image | ||
| |||
| Properties | |||
| C4H4O2 | |||
| Molar mass | 84.08 g mol−1 | ||
| Density | 1.09 g cm−3 | ||
| Melting point | −7 °C (19 °F; 266 K) | ||
| Boiling point | 127 °C (261 °F; 400 K) | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Diketene is an organic compound with the formula (CH2CO)2. It is formed by dimerization of ketene (IUPAC name: ethenone). Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry.[1] It is a colorless liquid.
Production
Ketene is generated by dehydrating acetic acid at 700–750 °C in the presence of triethyl phosphate as a catalyst or (in Switzerland and the CIS) by the thermolysis of acetone at 600–700 °C in the presence of carbon disulfide as a catalyst.[2]
- CH3CO2H → H2C=C=O + H2O (ΔH = +147 kJ/mol)
- CH3COCH3 → H2C=C=O + CH4
Reactions
Heating or irradiation with UV light [3] regenerates the ketene monomer:
- (CH2CO)2
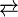 2 CH2CO
2 CH2CO
Alkylated ketenes also dimerize with ease and form substituted diketenes.
Diketene readily hydrolyses in water forming acetoacetic acid, its half-life in pure water is approximately 45 minutes a 25 °C at 2<pH<7.[4]
Certain diketenes with two aliphatic chains, such as alkyl ketene dimer (AKD), are used industrially to improve hydrophobicity in paper.
At one time acetic anhydride was prepared by the reaction of ketene with acetic acid:[2]
- H2C=C=O + CH3COOH → (CH3CO)2O (ΔH = −63 kJ/mol)
Acetoacetylation
Diketene also reacts with alcohols and amines to the corresponding acetoacetic acid derivatives. The process is sometimes called acetoacetylation. An example is the reaction with 2-aminoindane:[5]
Diketene is an important industrial intermediate used for the production of acetoacetate esters and amides as well as substituted 1-phenyl-3-methylpyrazolones. The latter are used in the manufacture of dyestuffs and pigments.[6] A typical reaction is:
- ArNH2 + (CH2CO)2 → ArNHC(O)CH2C(O)CH3
These acetoacetamides are precursors to arylide yellow and diarylide pigments.[7]
Safety
Despite its high reactivity as an alkylating agent, and unlike analogue β-lactones propiolactone and β-butyrolactone, diketene is inactive as a carcinogen, possibly due to the instability of its DNA adducts.[8]
References
- ↑ Beilstein E III/IV 17: 4297.
- ↑ 2.0 2.1 Arpe, Hans-Jürgen (2007-01-11), Industrielle organische Chemie: Bedeutende vor- und Zwischenprodukte (6th ed.), Weinheim: Wiley-VCH, pp. 200–1, ISBN 3-527-31540-3.
- ↑ Susana Breda, Igor Reva, and Rui Fausto (2012). "UV Induced Unimolecular Photochemistry of Diketene Isolated in Cryogenic Inert Matrices". J. Phys. Chem. A 116 (9): 2131–2140. doi:10.1021/jp211249k.
- ↑ Rafael Gómez-Bombarelli, Marina González-Pérez, María Teresa Pérez-Prior, José A. Manso, Emilio Calle and Julio Casado (2008). "Kinetic Study of the Neutral and Base Hydrolysis of Diketene". Phys. Org. Chem 22 (5): n/a. doi:10.1002/poc.1483.
- ↑ Kiran Kumar Solingapuram Sai, Thomas M. Gilbert, and Douglas A. Klumpp (2007). "Knorr Cyclizations and Distonic Superelectrophiles". J. Org. Chem. 72 (25): 9761–9764. doi:10.1021/jo7013092. PMID 17999519.
- ↑ Ashford's Dictionary of Industrial Chemicals, Third Edition, 2011, pages 3241-2.
- ↑ K. Hunger. W. Herbst "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a20_371
- ↑ Rafael Gómez-Bombarelli, Marina González-Pérez, María Teresa Pérez-Prior, José A. Manso, Emilio Calle and Julio Casado (2008). "Chemical Reactivity and Biological Activity of Diketene". Chem. Res. Toxicol. 21 (10): 1964–1969. doi:10.1021/tx800153j. PMID 18759502.