Dihydrogen bond
In chemistry, a dihydrogen bond is a kind of hydrogen bond, an interaction between a metal hydride bond and an OH or NH group or other proton donor. With a van der Waals radius of 1.2 Å, hydrogen atoms do not usually approach other hydrogen atoms closer than 2.4 Å. Close approaches near 1.8 Å, are however characteristic of dihydrogen bonding.[1]
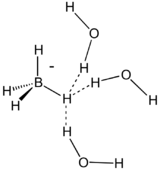
Boron hydrides
An early example of this phenomenon is credited to Brown and Heseltine.[2] They observed intense absorptions in the IR bands at 3300 and 3210 cm−1 for a solution of (CH3)2NHBH3. The higher energy band is assigned to a normal N−H vibration whereas the lower energy band is assigned to the same bond, which is interacting with the B−H. Upon dilution of the solution, the 3300 cm−1 band increased in intensity and the 3210 cm−1 band decreased, indicative of intermolecular association.
Interest in dihydrogen bonding was reignited upon the crystallographic characterization of the molecule H3NBH3. In this molecule, like the one studied by Brown and Hazeltine, the hydrogen atoms on nitrogen have a partial positive charge, denoted Hδ+, and the hydrogen atoms on boron have a partial negative charge, often denoted Hδ−.[3] In other words, the amine is a protic acid and the borane end is hydridic. The resulting B−H...H−N attractions stabilize the molecule as a solid. In contrast, the related substance ethane, H3CCH3, is a gas with a boiling point 285 °C lower. Because two hydrogen centers are involved, the interaction is termed a dihydrogen bond.
Formation of a dihydrogen bond is assumed to precede formation of H2 from the reaction of a hydride and a protic acid. A very short dihydrogen bond is observed in NaBH4·2H2O with H−H contacts of 1.79, 1.86, and 1.94 Å.[1]
Coordination chemistry
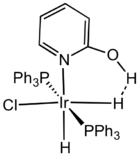
Protonation of transition metal hydride complexes is generally thought to occur via dihydrogen bonding.[4] This kind of H−H interaction is distinct from the H−H bonding interaction in transition metal complexes having dihydrogen bound to a metal.[5] Another so-called hydrogen hydrogen bond is postulated to exist in certain compounds between two neutral non-bonding hydrogen atoms. All of the types of dihydrogen bonds have been found in molecular aggregations.[6]
Notes
- ↑ 1.0 1.1 1.2 Custelcean, Radu; James E. Jackson (2001-07-01). "Dihydrogen Bonding: Structures, Energetics, and Dynamics". Chemical Reviews 101 (7): 1963–1980. doi:10.1021/cr000021b. PMID 11710237.
- ↑ Brown, M. P.; R. W. Heseltine (1968). "Co-ordinated BH3 as a proton acceptor group in hydrogen bonding". Chemical Communications (London) (23): 1551–1552. doi:10.1039/C19680001551.
- ↑ Crabtree, Robert H.; Per E. M. Siegbahn; Odile Eisenstein; Arnold L. Rheingold; Thomas F. Koetzle (1996-01-01). "A New Intermolecular Interaction: Unconventional Hydrogen Bonds with Element−Hydride Bonds as Proton Acceptor". Accounts of Chemical Research 29 (7): 348–354. doi:10.1021/ar950150s. PMID 19904922.
- ↑ Natalia V. Belkova, Elena S. Shubina, and Lina M. Epstein "Diverse World of Unconventional Hydrogen Bonds" Acc. Chem. Res., 2005, vol. 38, pp 624–631doi:10.1021/ar040006j
- ↑ Kubas, Gregory J. (2001-08-31). Metal Dihydrogen and -Bond Complexes - Structure, Theory, and Reactivity (1 ed.). Springer. ISBN 0-306-46465-9.
- ↑ Bakhmutov, Vladimir. I. Dihydrogen bonds: Principles, Experiments and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, 2008. ISBN 9780470180969
| ||||||||||||||||||||||||||||||||||||||||||||