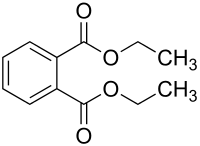
Diethyl phthalate
 | |
 | |
| Names | |
|---|---|
| Other names
DEP, Diethyl ester of phthalic acid, Ethyl phthalate | |
| Identifiers | |
| 84-66-2 | |
| ChEBI | CHEBI:34698 |
| ChEMBL | ChEMBL388558 |
| ChemSpider | 13837303 |
| |
| Jmol-3D images | Image |
| KEGG | D03804 |
| PubChem | 6781 |
| |
| UNII | UF064M00AF |
| Properties | |
| C12H14O4 | |
| Molar mass | 222.24 g/mol |
| Appearance | colourless, oily liquid |
| Density | 1.12 g/cm3 at 20 °C |
| Melting point | −40.5 °C (−40.9 °F; 232.7 K) |
| Boiling point | 295 °C (563 °F; 568 K) |
| 1080 mg/L at 25 °C | |
| log P | 2.42 |
| Vapor pressure | 0.002 mmHg (25°C)[2] |
| Hazards | |
| NFPA 704 | |
| Flash point | 161.1 °C (322.0 °F; 434.2 K)[2] |
| Explosive limits | 0.7%-?[2] |
| LD50 (Median lethal dose) |
8600 mg/kg (Rat) |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[2] |
| REL (Recommended) |
TWA 5 mg/m3[2] |
| IDLH (Immediate danger) |
N.D.[2] |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Diethyl phthalate (DEP) is a phthalate ester, namely the diethyl ester of phthalic acid. It is a clear substance that is liquid at room temperature and is only slightly more dense than liquid water. It has a faint, disagreeable odor and can be transferred from the plastics that contain it.[3] When burned, DEP produces toxic gases.[4]
Since the compound is a suitable solvent for many organic molecules, it is often used to bind cosmetics and fragrances.[5] Other industrial uses include plasticizers, detergent bases and aerosol sprays.[6] Because of the frequent dermal exposure of humans to the chemical, the question of toxicity is crucial. Several studies suggest that DEP can cause damage to the nervous system as well as to the reproductive organs in males and females.[7][8][9]
Exposure
Due to their use as plasticizers, diethyl phthalates are ubiquitous in the environment, especially near places of production and use. Biodegradation through microbially-mediated processes can result in products that can potentially harm microorganisms.[10] There is also general evidence of widespread human exposure.[11][12][13][14] Non-occupational exposure results from the diet, for example phthalate-coated medicines and nutritional supplements, and through consumer products.[14] High occupational exposure was observed in workers directly manufacturing plasticizers.[15] Studies suggest a high correlation between air and urine sample concentrations of short side-chain phthalates such as DEP, making inhalation an important route of exposure.[12][15]
Structure and reactivity
Diethyl phthalate, or o-Benzenedicarboxylic acid diethyl ester consists of a benzene ring with two carboxylic acid ethyl esters attached to it in the ortho (1,2) pattern.[1] It is a highly conjugated system, as the pi-cloud on the benzene ring, the p-orbitals on the carbonyl atoms and the lone pairs on the oxygens are all conjugated. The substituents are meta-directing,[16] and they are ortho to each other, so all positions in the ring are more or less equally deactivated. Diethyl phthalate is likely to undergo biodegradation in the environment.[17] Abiotic degradation processes such as hydrolysis, oxidation, and photolysis are unlikely to play significant roles in the environmental fate of diethyl phthalate.
Synthesis
Diethyl phthalate is produced by the reaction of phthalic anhydride with ethanol in the presence of a catalytic amount of concentrated sulfuric acid.[1] Phthalic anhydride is produced by either the oxo process or the Ald-Ox process from ethanol and the oxidation of naphthalene or o-xylene.[18] The purity of manufactured phthalate esters is reportedly between 99.70% and 99.97% with the main impurities being isophthalic acid, terephthalic acid, and maleic anhydride.[18]

Metabolism
Diethyl phthalate is hydrolyzed to monoester, monoethyl phthalate and ethanol after oral administration in the lumen of the gastrointestinal tract or in the intestinal mucosal cells. Hydrolysis of DEP also takes place at the kidney and liver after systemic absorption. After tissue distribution throughout the body, DEP accumulates in the liver and kidney. The metabolites are excreted in the urine.[5] DEP is metabolized by carboxyl esterase, which is synthesized in the human liver. In vitro studies show that DEP reduces the glucuronyl transferase activity. It was also observed that the activity of peroxisomal enzyme carnitine acetyl transferase is increased in cultures of rat liver cells.[5] Furthermore DEP induces the enzyme activity of catalase, which leads to hepatic peroxisome proliferation and possibly causes hyperplasia.[19]
Biodegradation
Biodegradation by microorganisms
Biodegradation of DEP in soil occurs by sequential hydrolysis of the two diethyl chains of the phthalate to produce monoethyl phthalate, followed by phthalic acid. This reaction occurs very slowly in an abiotic environment. Thus there exists an alternative pathway of biodegradation which includes transesterification or demethylation by microorganisms, if the soil is also contaminated with methanol, that would produce another three intermediate compounds, ethyl methyl phthalate, dimethyl phthalate and monomethyl phthalate. This biodegradation has been observed in several soil bacteria.[10] Some bacteria with these abilities have specific enzymes involved in the degradation of phthalic acid esters such as phthalate oxygenase, phthalate dioxygenase, phthalate dehydrogenase and phthalate decarboxylase.[20] The developed intermediates of the transesterification or demethylation, ethyl methyl phthalate and dimethyl phthalate, enhance the toxic effect and are able to disrupt the membrane of microorganisms.[10]
Biodegradation by mammals
Recent studies show that DEP, a phthalic acid ester (PAE), is enzymatically hydrolyzed to its monoesters by pancreatic cholesterol esterase (CEase) in pigs and cows. These mammalian pancreatic CEases have been found to be nonspecific for degradation in relation to the diversity of the alkyl side chains of PAEs. .[20]
Toxicity
Little is known about the chronic toxicity of diethyl phthalate, but existing information suggests only a low toxic potential.[21] Studies suggest that some phthalates affect male reproductive development via inhibition of androgen biosynthesis. In rats, for instance, repeated administration of DEP results in loss of germ cell populations in the testis. However, diethyl phthalate doesn't alter sexual differentiation in male rats.[11][22][23][24] Dose response experiments in fiddler crabs have shown that seven-day exposure to diethyl phthalate at 50 mg/L significantly inhibited the activity of chitobiase in the epidermis and hepatopancreas.[25] Chitobiase plays an important role in degradation of the old chitin exoskeleton during the pre-moult phase.[26]
Teratogenicity
When pregnant rats where treated with diethyl phthalate, it became evident that certain doses caused skeletal malformations, whereas the untreated control group showed no resorptions. The amount of skeletal malformations was highest at highest dose.[27] In a following study it was found that both phthalate diesters and their metabolic products were present in each of these compartments, suggesting that the toxicity in embryos and fetuses could be the result of a direct effect.[28]
Future investigation
Some data suggest that exposure to multiple phthalates at low doses significantly increases the risk in a dose additive manner.[29][30][31] Therefore, the risk from a mixture of phthalates or phthalates and other anti-androgens, may not be accurately assessed studying one chemical at a time. The same may be said about risks from several exposure routes together. Humans are exposed to phthalates by multiple exposure routes (predominantly dermal), while toxicological testing is done via oral exposure.[9]
References
- ↑ 1.0 1.1 1.2 "Chemical Information Profile for Diethyl Phthalate" (PDF). Integrated Laboratory Systems, Inc. Retrieved 3 March 2009.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "NIOSH Pocket Guide to Chemical Hazards #0213". National Institute for Occupational Safety and Health (NIOSH).
- ↑ ATSDR ToxFAQ for Diethyl phthalate
- ↑ ICSC:NENG0258 (International Chemical Safety Card for 1,2-Benzenedicarboxylic acid diethyl ester)
- ↑ 5.0 5.1 5.2 Api, A.M. (2001). "Toxicological profile of diethyl phthalate: a vehicle for fragrance and cosmetic ingredients". Food and Chemical Toxicology 39 (2): 97–108. doi:10.1016/S0278-6915(00)00124-1.
- ↑ Ghorpade, N.; Mehta, V.; Khare, M. (2002). "Toxicity Study of Diethyl Phthalate on Freshwater Fish Cirrhina mrigala". Ecotoxicology and Environmental Safety 53 (2): 255–258. doi:10.1006/eesa.2002.2212.
- ↑ Miodovnik, A. (March 2011). "Endocrine disruptors and childhood social impairment". Neurotoxicology 32 (2): 261–267. doi:10.1016/j.neuro.2010.12.009. PMC 3057338. PMID 21182865.
- ↑ Ivelisse Colón et al. (2000). "Identification of Phthalate Esters in the Serum of Young Puerto Rican Girls with Premature Breast Development". Environmental Health Perspectives 108: 895–900. doi:10.1289/ehp.00108895. PMC 2556932. PMID 11017896.
- ↑ 9.0 9.1 Shanna H. Swan (2008). "Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans". Environmental Research 108 (2): 177–184. Bibcode:2008ER....108..177S. doi:10.1016/j.envres.2008.08.007. PMC 2775531. PMID 18949837.
- ↑ 10.0 10.1 10.2 Cartwright, C.D. (March 2000). "Biodegradation of diethyl phthalate in soil by a novel pathway". FEMS Microbiology Letters 186 (1): 27–34. doi:10.1016/S0378-1097(00)00111-7.
- ↑ 11.0 11.1 Antonia M. Calafat and Richard H. McKee (2006). "Integrating Biomonitoring Exposure Data into the Risk Assessment Process: Phthalates [Diethyl Phthalate and Di(2-ethylhexyl) Phthalate] as a Case Study". Environmental Health Perspectives 114 (11): 1783–1789. doi:10.1289/ehp.9059.
- ↑ 12.0 12.1 Adibi, J.J. (2003). "Prenatal Exposures to Phthalates among Women in New York City and Krakow, Poland". Environmental Health Perspectives 111 (14): 1719–1722. doi:10.1289/ehp.6235. PMC 1241713. PMID 14594621.
- ↑ Blount, B.C. (July 2000). "Quantitative Detection of Eight Phthalate Metabolites in Human Urine Using HPLC-APCI-MS/MS". Anal. Chem. 72 (17): 4127–4134. doi:10.1021/ac000422r.
- ↑ 14.0 14.1 Schettler, Ted (February 2006). "Human exposure to phthalates via consumer products". International Journal of Andrology 29 (1): 134–139. doi:10.1111/j.1365-2605.2005.00567.x.
- ↑ 15.0 15.1 Hines, Cynthia J. (2008). "Urinary Phthalate Metabolite Concentrations among Workers in Selected Industries: A Pilot Biomonitoring Study". The Annals of Occupational Hygiene 53 (1): 1–17. doi:10.1093/annhyg/men066.
- ↑ Jones, Jr., Maitland (2005). Organic Chemistry. New York: W.W Norton & Company. p. 715.
- ↑ U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry "Toxicological profile for Diethyl Phthalate" June 1995
- ↑ 18.0 18.1 Peakall D.B. (1975). "Phthalate esters: Occurrence and biological effects.". Residue Rev 54: 1–41. doi:10.1007/978-1-4612-9857-1_1.
- ↑ Timbrell, J. (2009). Principles of Biochemical Toxicology. London: Informa Healthcare. pp. 179, 200–201.
- ↑ 20.0 20.1 Saito, T.; Peng, H.; Tanabe, R.; Nagai, K.; Kato, K. (December 2010). "Enzymatic hydrolysis of structurally diverse phthalic acid esters by porcine and bovine pancreatic cholesterol esterases.". Chemosphere 81 (1). doi:10.1016/j.chemosphere.2010.08.020.
- ↑ J. Autian (1973). "Toxicity and health threats of phthalate esters: review of the literature". Environmental Health Perspectives 4: 3–25. doi:10.2307/3428178. PMC 1474854. PMID 4578674.
- ↑ Paul M. D. Foster et al. (1980). "Study of the testicular effects and changes in zinc excretion produced by some n-alkyl phthalates in the rat". Toxicology and Applied Pharmacology 54 (3): 392–398. doi:10.1016/0041-008X(80)90165-9. PMID 7394794.
- ↑ P. M. D. Foster et a. (1981). "Studies on the testicular effects and zinc excretion produced by various isomers of monobutyl-o-phthalate in the rat". Chemico-Biological Interactions 34 (2): 233–238. doi:10.1016/0009-2797(81)90134-4.
- ↑ L. Earl Gray Jr. et al. (2000). "Perinatal Exposure to the Phthalates DEHP, BBP, and DINP, but Not DEP, DMP, or DOTP, Alters Sexual Differentiation of the Male Rat". Toxicological Sciences 58 (2): 350–365. doi:10.1093/toxsci/58.2.350. PMID 11099647.
- ↑ "Effects of exposure to diethyl phthalate, 4-(tert)-octylphenol, and 2,4,5-trichlorobiphenyl on activity of chitobiase in the epidermis and hepatopancreas of the fiddler crab, Uca pugilator". Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology 122 (1): 115–120. 1999. doi:10.1016/S0742-8413(98)10093-2.
- ↑ M. A. Baars & S.S. Oosterhuis, "Free chitobiase, a marker enzyme for the growth of crustaceans", NIOZ Annual Report 2006 (PDF), Royal Netherlands Institute for Sea Research, Texel, pp. 62–64
- ↑ A. R. Singh, W. H. Lawrence, J. Autian (1972). "Teratogenicity of Phthalate Esters in Rats". Journal of Pharmaceutical Sciences 61 (1): 51–55. doi:10.1002/jps.2600610107.
- ↑ A. R. Singh, W. H. Lawrence, J. Autian (1975). "Maternal-Fetal transfer of 14C-Di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats". Journal of Pharmaceutical Sciences 64 (8): 1347–1350. doi:10.1002/jps.2600640819.
- ↑ L. Earl Gray Jr. et al. (2006). "Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals". International Journal of Andrology 29 (1): 96–104. doi:10.1111/j.1365-2605.2005.00636.x.
- ↑ Kembra L. Howdeshell et al. (2008). "A Mixture of Five Phthalate Esters Inhibits Fetal Testicular Testosterone Production in the Sprague-Dawley Rat in a Cumulative, Dose-Additive Manner". Toxicological Sciences 105 (1): 153–165. doi:10.1093/toxsci/kfn077.
- ↑ Kembra L. Howdeshell et al. (2008). "Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats". Environmental Research 108 (2): 168–176. Bibcode:2008ER....108..168H. doi:10.1016/j.envres.2008.08.009.
