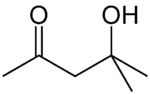
Diacetone alcohol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4-Hydroxy-4-methyl-pentan-2-one | |
| Other names
2-Pentanone, 4-hydroxy-4-methyl; 4-Hydroxy-2-keto-4-methylpentane; Diacetone alcohol | |
| Identifiers | |
| 123-42-2 | |
| ChEBI | CHEBI:55381 |
| ChemSpider | 13838151 |
| |
| Jmol-3D images | Image |
| RTECS number | SA9100000 |
| |
| Properties | |
| Molecular formula |
C6H12O2 |
| Molar mass | 116.16 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 0.938 g/cm3 |
| Melting point | −47 °C (−53 °F; 226 K) |
| Boiling point | 166 °C (331 °F; 439 K) |
| moderate | |
| Solubility | most organic solvents |
| Refractive index (nD) |
1.4235 |
| Hazards | |
| Main hazards | Flammable |
| R-phrases | R36 |
| S-phrases | S24/25 |
| Flash point | 52 °C; 125 °F; 325 K |
| Explosive limits | 1.8%-6.9%[1] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 50 ppm (240 mg/m3)[1] |
| Related compounds | |
| Related compounds |
Acetone methyl isobutyl ketone |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Diacetone alcohol is a chemical compound with the formula CH3C(O)CH2C(OH)(CH3)2, sometimes called DAA. This liquid is a common synthetic intermediate used for the preparation of other compounds, and is also used as a solvent.
It occurs naturally in Sleepy grass (Achnatherum robustum).
Synthesis
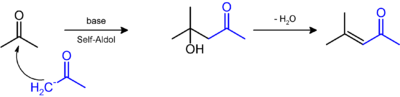
First identified by Heintz,[2] one standard laboratory preparation of DAA entails the Ba(OH)2-catalyzed condensation of two molecules of acetone.[3]
It undergoes dehydration to give the α,β-unsaturated ketone, mesityl oxide:[4] Hydrogenation of mesityl oxide gives the industrial solvent, methyl isobutyl ketone ("MIBK").
Uses
It is used in cellulose ester lacquers, particularly of the brushing type, where it produces brilliant gloss and hard film and where its lack of odor is desirable. It is used in lacquer thinners, dopes, wood stains, wood preservatives and printing pastes; in coating compositions for paper and textiles; permanent markers;[5] in making artificial silk and leather; in imitation gold leaf; in celluloid cements; as a preservative for animal tissue; in metal cleaning compounds; in the manufacture of photographic film; and in hydraulic brake fluids, where it is usually mixed with an equal volume of castor oil.
References
- ↑ 1.0 1.1
- ↑ Heintz, Ann. 169, 114 (1873)
- ↑ Conant, J. B.; Tuttle, N. (1941). "Diacetone Alcohol". Org. Synth.; Coll. Vol. 1, p. 199
- ↑ Conant, J. B.; Tuttle, N. (1941). "Mesityl Oxide". Org. Synth.; Coll. Vol. 1, p. 345
- ↑ MSDS: Sharpie Chisel Tip Permanent Marker - "Household products database" US Department of Health and Human Services