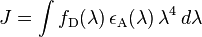Dexter electron transfer
_transfer.png)
Dexter electron transfer (also called Dexter electron exchange and Dexter energy transfer) is a fluorescence quenching mechanism in which an excited electron is transferred from one molecule (a donor) to a second molecule (an acceptor) via a non radiative path.[1][2] This process requires a wavefunction overlap between the donor and acceptor,[3] which means it can only occur at short distances; typically within 10 Å.[4] The excited state may be exchanged in a single step, or in two separate charge exchange steps.
The Dexter energy transfer rate, 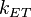 , is indicated by the proportionality
, is indicated by the proportionality
where  is the separation of the donor from the acceptor,
is the separation of the donor from the acceptor, 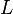 is the sum of the Van der Waals radii of the donor and the acceptor, and
is the sum of the Van der Waals radii of the donor and the acceptor, and 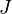 is the spectral overlap integral defined by
is the spectral overlap integral defined by
This short range energy transfer process was first theoretically proposed by D. L. Dexter in 1951.[5]
See also
References
- ↑ Clifford B. Murphy, Yan Zhang, Thomas Troxler, Vivian Ferry, Justin J. Martin, and Wayne E. Jones, Jr. (2004). "Probing Förster and Dexter Energy-Transfer Mechanisms in Fluorescent Conjugated Polymer Chemosensors". J. Phys. Chem. B 108 (5): 1537–1543. doi:10.1021/jp0301406.
- ↑ Alex Adronov and Jean M. J. Fréchet (2000). "Light-harvesting dendrimers". Chem. Commun.: 1701–1710. doi:10.1039/B005993P.
- ↑ "Dexter (electron exchange) excitation transfer". goldbook.iupac.org. Retrieved 8 July 2014.
- ↑ "Dexter Energy Transfer". chemwiki.ucdavis.edu. Retrieved 8 July 2014.
- ↑ D. L. Dexter (1951). "A Theory of Sensitized Luminescence in Solids". J. Chem. Phys. 21: 836–850. doi:10.1063/1.1699044.
![k_{ET} \varpropto J \mathrm{exp}\left [ \frac{-2r}{L} \right ]](../I/m/f518bc5ce50b83acb34a0dd150d9fdc0.png)